Discovery upends 100-year-old brain cell theory taught in scientific textbooks
Brains run on timing. A fraction of a second can decide whether one message arrives before another and changes what a circuit does. Signals travel along axons – the thin extensions of brain cells (neurons) that act like wires.
Textbooks have long shown many unmyelinated axons as smooth tubes, meaning that the electrical signal must travel continuously along the entire membrane, rather than jumping from node to node.
Careful measurements now show a different picture: a repeating rhythm of small bulges and narrow links – a pattern that acts as a built‑in control for speed.
Unmyelinated axons often don’t look like plain cylinders. They carry a repeating geometry that toggles between slightly wider and narrower segments.
That geometry alters how fast electrical impulses move. And because the shape can shift with everyday activity, it forms a real‑time dial for signal timing.
Axons, membranes, and pearls
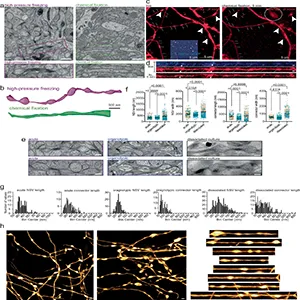
Researchers preserved brain tissue with high‑pressure freezing to keep delicate membranes near their living state.
In those samples, unmyelinated axons routinely showed tiny, non-synaptic swellings only about 8 millionths of an inch across (0.2 micrometers) – a few hundred times thinner than a human hair.
Between the swellings ran a thinner “connector” segment, about 2.4 millionths of an inch (0.06 micrometers). The axon alternated between the two along its length – “pearls on a string.”
They confirmed the pattern in living tissue using super‑resolution microscopy, showing it was not a freezing artifact.
Typical chemical fixatives, a standard approach for electron microscopy, smoothed the axons and made the pearls hard to spot. That helps explain why the field overlooked this structure for years.
Pearl-shaped axon membranes
Cell membranes behave like ultra‑thin, flexible surfaces that must balance bending and tension. The researchers modeled axon membranes with the Helfrich–Canham framework, a physics approach that weighs bending forces, membrane tension, and pressure differences across the membrane.
At the tiny diameters found in these axons, those forces favor a beaded pattern rather than a uniform tube.
In that model, the pearl‑and‑connector geometry emerges naturally as a low‑energy state. It does not require special scaffolds at every bead. Ordinary membrane mechanics can make the shape appear and persist.
Seeing a pattern is one thing. Showing that mechanics set that pattern is another matter completely. The team nudged membrane mechanics three ways and watched the geometry and speed respond. Each push shifted pearl dimensions in the directions predicted by the model.
They also recorded how fast a composite signal travels along bundles of axons. This “fiber volley” (a combined signal recorded from many axons) slowed after cholesterol removal by 30%.
After myosin inhibition, it rose by 17%. Those shifts link nanoscale shape to bundle‑level conduction.
Activity reshapes membranes
Neurons that fired rapidly in repeated bursts showed swift structural changes. Within minutes, the pearls grew by about 17% in width and about 8% in length, while the connectors held their size.
At the same time, a cholesterol sensor on the cell surface reported about a 45% drop in membrane cholesterol after sustained activity.
Cholesterol tunes membrane stiffness and organization, so that drop shifts the mechanical balance and changes pearl geometry. The result is a small but meaningful change in conduction speed that emerges from everyday firing patterns.
This is plasticity without adding or removing myelin or changing synapse number. The membrane itself adjusts shape and, with it, timing.
“Wait – aren’t bulges in axons a bad sign?” In injured or degenerating neurons, big “beads” can appear as the cell breaks down. That is not what shows up here. These features are much smaller, occur under healthy conditions, and do not align with synapses.
The team watched them in living tissue and found they remained stable during imaging sessions, which argues against the idea that they are large cargo getting stuck and clogging traffic. This pattern looks like a structural element of normal axons.
Tiny axon membrane changes matter
Electrical signals in axons follow cable theory. Widening a segment lowers internal resistance and raises membrane capacitance. Narrowing does the opposite.
Repeat the pattern along a cable and you create a scaffold that shapes how fast the signal moves. The pearl‑and‑connector rhythm acts as a “fine‑tuning knob” for conduction speed.
Because the membrane’s composition and tension can shift with activity and pharmacology, the knob turns on a timescale relevant to behavior.
That offers a clear way for unmyelinated pathways to adjust timing for tasks that depend on precise delays, such as sensory coding, rhythm generation, and certain forms of learning.
What happens next?
Future studies can map how membrane lipids, cytoskeletal rings, and membrane‑tethering proteins coordinate to set geometry in different circuits.
The hypothesis is straightforward: change the mechanical balance and you change bead size, connector width, and conduction speed. Testing that across regions and species will tell us how universal this control really is.
Unmyelinated axons are not smooth wires. They carry a nanoscale pattern whose beads and links reflect the physics of a living membrane.
Small shifts in membrane composition or tension retune the speed of electrical messages that travel millimeters through tissue.
That connection between mechanics and timing widens the menu of ways the brain can adjust itself, and it does so using tools – lipids, motors, and water flux – that cells already use every day.
The full study was published in the journal Nature Neuroscience.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













