Space travel pushes human stem cells into an accelerated aging pattern
Space scientists report that spending time in microgravity makes certain blood-forming stem cells in humans act “old” in a much faster timeframe than they should.
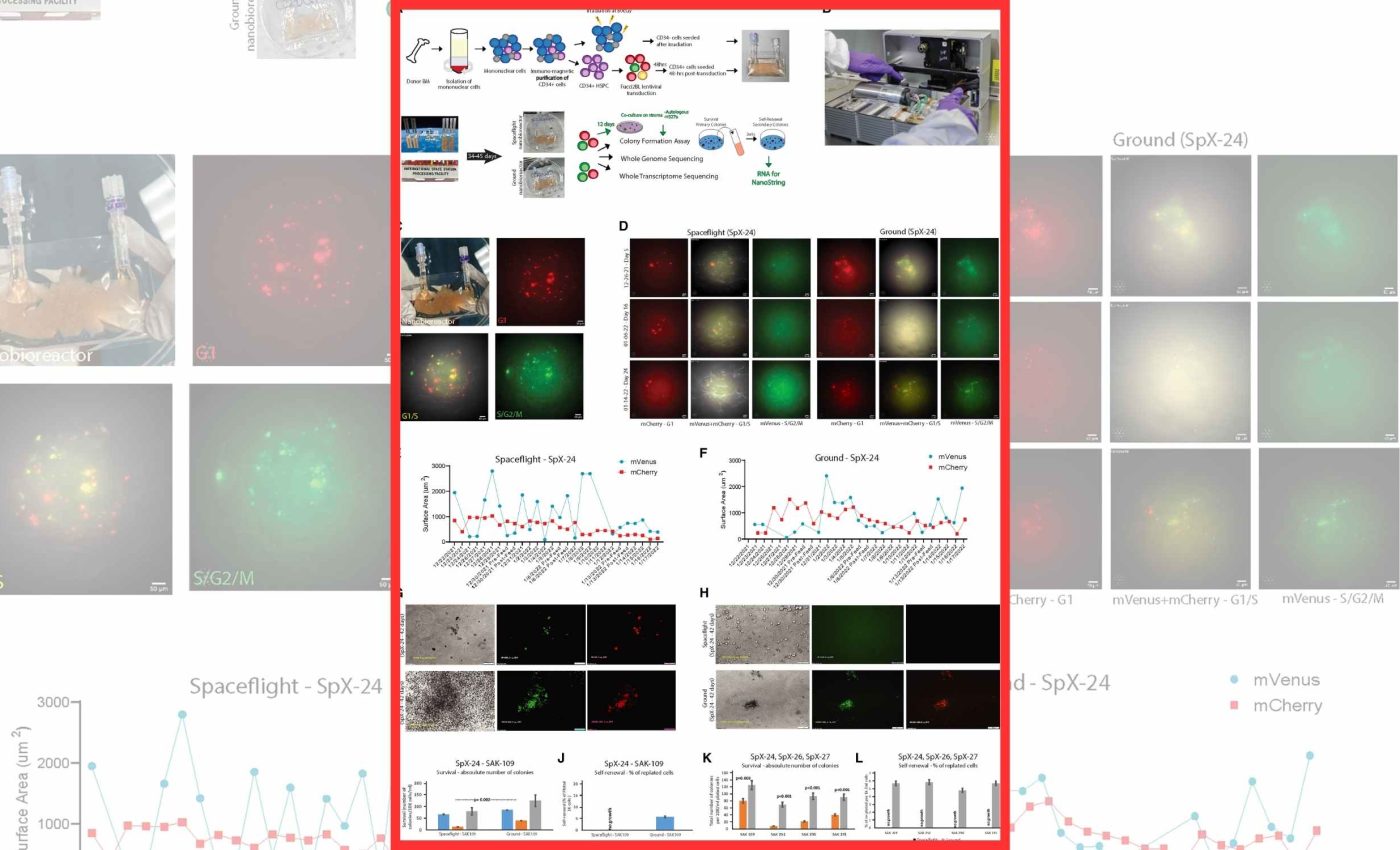
In a recent study, human bone marrow cells spent 32 to 45 days aboard the International Space Station and came home showing classic signs of accelerated aging.
The team found that these cells burned through energy, lost their usual rest state, and switched on normally silent stretches of DNA. The work was based in San Diego and flown on four SpaceX missions to the station.
Stem cells and space travel
The work was led by Catriona Jamieson, M.D., Ph.D., at the University of California San Diego (UCSD). Her research focuses on how stress drives stem cells toward disease and how to stop that slide.
Researchers used palm-size bioreactors to grow hematopoietic stem and progenitor cells, blood-making cells that renew the immune system, in orbit. The cells were tracked with on-board imaging and returned for deep genetic analysis on Earth.
Researchers noted in a report that stem cells in orbit lose some of their usual function. They explained that these cells show reduced capacity to renew or regenerate, which matters for anyone spending long periods in space.
The cells were more awake than they should be, and they struggled to slip back into dormancy. That loss of rest is a hallmark of aging in the blood system.
Repetitive DNA and microgravity
The study found a surge in activity from repetitive elements, repeated DNA sequences from ancient viral insertions that usually stay quiet. This hidden “dark genome” activity rose in space, a signal that cells were under unusually strong stress.
Scientists also saw changes in inflammatory signals and mitochondria. Those shifts are part of the same stress web that nudges cells toward aging states.
Microgravity, very weak gravity in orbit that creates weightlessness sensations, can disturb how cells sense force and organize themselves. That environment, plus space radiation, appears to push blood stem cells to cycle faster.
Fast cycling burns metabolic reserves and interrupts normal repair routines. Over weeks, the pattern resembles what happens over many years on Earth.
Clues from telomeres and mutations
The team detected a downturn in telomeres, DNA caps that protect chromosome ends and shorten with age. Cells also showed a tilt in the expression of genes that maintain telomeres, hinting at stress on genome upkeep.
They found single-letter DNA changes with a C-to-T pattern tied to enzymes that edit nucleic acids under inflammatory pressure.
Some cells carried mutations associated with clonal hematopoiesis, age-linked blood cell clones that raise risks for cardiovascular disease and leukemia later in life.
When space-exposed cells were cultured on a young, healthy support layer, some of the damage eased. Self-renewal improved, and stress-related gene activity shifted toward a calmer state.
That recovery suggests we can design countermeasures that restore rest and repair pathways. It also hints at screening methods to see who is most resilient before flight.
Links with astronaut biology
A landmark year-long astronaut study showed shifts in gene activity, immune responses, and telomere dynamics tied to time in orbit. Many changes faded after landing, but not all did.
More recently, an open resource called the Space Omics and Medical Atlas integrates multi-omic astronaut data across missions.
That resource points to common threads of mitochondrial stress, inflammation, and DNA stability that match what was seen in the stem cell work.
Blood and immune systems must stay sharp on months-long journeys. If stem cells tire early, crews could face higher infection risks, slower wound healing, and more inflammation.
These findings make a case for on-mission monitoring of stem cell health. They also support testing of drugs that stabilize cell dormancy and block harmful DNA activation.
The team saw signals from enzymes that edit RNA and DNA during stress surges. Those editors help control viral-like sequences but can also add mutations when overactive.
The strongest space signals pointed to a push-pull between editing enzymes and the dark genome. That tug of war helps explain both the mutational pattern and the rise of repetitive element transcripts.
Stem cell lessons from space travel
Future missions will sample cells during flight to map changes as they happen. That will help disentangle the effects of radiation bursts, microgravity, and sleep cycles.
On Earth, the same platform can model cancer-prone aging in the blood system. Insights could speed therapies for patients whose stem cells show similar stress.
Short station stays changed fundamental traits of blood stem cells. The pattern points to earlier aging of the system that defends us from infection and cancer.
At the same time, parts of the damage were reversed under youthful conditions. That gives engineers and physicians a target for countermeasures before we go farther from Earth.
The study is published in Cell Stem Cell.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













