
Scientists may have found a near-limitless energy source that could power Earth forever
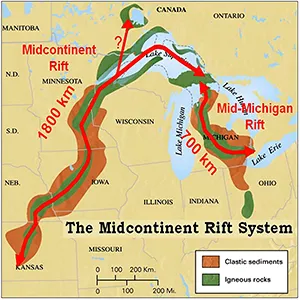
The Midcontinent Rift is an ancient crack in the ground that started to open across the middle of North America about 1.1 billion years ago. Over time, this rift became home to magma, water, metals, and even natural hydrogen.
Tracing this arc to Lake Superior, you can still find the rift rocks and metals near the surface. Some formed straight from magma as it cooled; others came later when hot, mineral-laden water moved through cracks and left metals behind.
For decades, rift exploration focused on hydrothermal energy. Lately, however, interest has swung back toward magmatic deposits, with copper-nickel sulfides explored in Minnesota’s Duluth Complex, and nickel coming out of the Eagle Mine in Michigan.
That deep history raises a current energy question: can those iron‑bearing rocks in the Midcontinent Rift, interacting with water underground, make usable and natural hydrogen gas?
The idea sounds great on paper, but it takes boots on the ground to get real answers – chemistry, rock structure, fluids, microbes, and engineering working together.
Natural hydrogen in the rift
People often hear about “green hydrogen,” made by splitting water at facilities powered by clean electricity. That route works, but it needs major power and equipment.
There’s another category: natural hydrogen, sometimes called “white hydrogen.”
It can form when water reacts with certain minerals in the subsurface and breaks apart to release hydrogen. Earth supplies the ingredients and the setting; no facility is needed to start the reaction.
Those reactions can continue as long as water, reactive minerals, heat, and open pathways keep interacting. This resource doesn’t behave like a fixed deposit. It can renew as the system cycles.
Rift rocks and gases
The rift contains thick stacks of mafic rock – basalts and related intrusions rich in iron and magnesium. Those minerals drive water‑rock reactions that free hydrogen.
Rifting also cracked the crust, producing fractures and faults that store fluids in some zones and allow movement in others.
Over long periods, pulses of heat and mineralizing fluids opened some pathways and sealed others. That complexity sets up places where hydrogen could form, migrate, and collect.
Porous layers often sit beneath tighter rocks that act as seals. Where a seal overlies a porous layer, gas can accumulate below. The rift’s varied geology provides many such pairs at a range of depths.
A research group in Nebraska set out to test whether hydrogen forms in measurable amounts where rift rocks lie within reach of modern drilling.
They want to know whether the gas accumulates in traps or escapes through leaks, and whether microbes consume it before it can build up.
Digging down deep
The team drilled a test well into rift rocks at a depth of several thousand feet. That depth sits in a “sweet spot” for exploration: deep enough for natural trapping and isolated from shallow groundwater, yet still practical for drilling and monitoring with standard tools.
They analyze gas compositions and flow behavior, test how hydrogen moves through fractures and pores, and check whether the gas dissolves into salty formation water or holds onto mineral surfaces.
If it dissolves or adsorbs too readily, capture becomes harder. If it moves too freely, it may leak away before anyone can gather it.
Microbial life can live in deep rock without sunlight. Microbes survive on chemical energy, and some microbes “eat” hydrogen.
The researchers look for those organisms in samples and measure how fast they consume hydrogen at the temperatures, pressures, and salinities found at depth.
If the community thrives, it could strip out hydrogen as fast as it forms. If chemistry or heat limits the microbes, more hydrogen could persist.
What they found so far
So far, the data is promising. Scientists at University of Nebraska-Lincoln (UNL) believe it is possible the geomechanical and biogeochemical conditions in the rift limit the loss and consumption of this naturally generated hydrogen
This fact could leave trapped hydrogen at an economically meaningful scale in the mid-continent subsurface.
“It could be deep enough to be stored but shallow enough that we can access it,” said Karrie Weber, professor of Earth and atmospheric sciences and biological sciences at UNL and another project investigator. “The geology is in our favor.”
How the rift makes hydrogen
Iron‑rich minerals such as olivine and pyroxene can react with water in ways that split water molecules. In simple terms, electrons move, bonds break, and hydrogen molecules form.
Fresh mineral surfaces react fastest, so fractures that expose new rock matter most. Temperature sets reaction speed, and pressure influences how much hydrogen dissolves into brines versus rising as a gas.
Other natural rift pathways also produce hydrogen.
Radiolysis – the breakup of water molecules by natural radiation in rock – can generate hydrogen. Interaction of ultramafic rocks with water, often called serpentinization, contributes as well.
The rift’s mafic and related rocks offer multiple routes to hydrogen, with local conditions deciding which process dominates.
Importance of hydrogen
Hydrogen shines bright as an energy source. In fuel cells, it produces electricity and water. In high‑temperature applications, it can step in for fossil fuels and cut carbon dioxide emissions.
Heavy trucks, some industrial furnaces, and backup power systems already move in this direction.
If natural hydrogen proves available at scale and reasonable cost, it could complement manufactured supplies and reduce the need for new facilities and the electricity they require.
If subsurface rift systems continue to generate hydrogen over time, production could be steady enough to serve as baseload (a steady, always‑on, limitless supply).
Pulling hydrogen from the rift
Clear signs that this plan is legitimate would include measurable hydrogen at depth, stable concentrations over time, and evidence of trapping beneath reliable seals.
Wells would need to sustain flow without rapid decline. Surface equipment would need to separate hydrogen from other gases and handle it efficiently. Proven monitoring methods would have to track the system and confirm integrity year after year.
Meeting those requirements would indicate a resource capable of supporting fuel cells, turbines, or process heat without trading short‑term gains for long‑term risks.
The current work tests whether those ingredients add up to a practical resource that wells can reach and manage safely.
The effort ties chemistry, geology, microbiology, and engineering into one program aimed at clear answers.
Whatever the ultimate outcome – production or a map of places not to drill – the result improves how we think about clean energy options shaped by Earth’s own chemistry.
The full study was published in the journal Ore Geology Direct.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













