
Common fish found to have a very uncommon row of teeth on its forehead
For more than a century, biologists have treated teeth as strictly oral equipment – no matter the animal. A new study of the spotted ratfish (a shark relative commonly found in Puget Sound) blows that assumption wide open.
Adult males of this shark species grow rows of hooked, barbed “teeth” on a cartilaginous forehead appendage called a tenaculum, which they flare to spar with rivals and grasp mates during underwater courtship.
“This absolutely spectacular feature flips the long-standing assumption in evolutionary biology that teeth are strictly oral structures,” said lead author Karly Cohen, a postdoctoral researcher at the University of Washington.
“The tenaculum is a developmental relic, not a bizarre one-off, and the first clear example of a toothed structure outside the jaw.”
Shark’s cousin has tenaculum
Spotted ratfish are chimaeras, a branch of cartilaginous fishes that split from sharks long ago.
They’re compact – about two feet long – with a whip-like tail. In males, the unmistakable forehead “peanut” can extend into a spiny hook.
Like sharks and rays, many chondrichthyan fishes are armored in tooth-like skin structures called denticles.
Ratfish are oddballs: aside from denticles on their pelvic claspers (another mating aid), their skin is mostly bare.
That contrast made scientists wonder what, exactly, the tenaculum spines are. Are they denticles in disguise – or true teeth transplanted to the head?
“Sharks don’t have arms, but they need to mate underwater,” Cohen said. “So a lot of them have developed grasping structures to connect themselves to a mate during reproduction.”
Ratfish have two: the forehead tenaculum and pelvic claspers.
Competing theories on tenaculum
The researchers laid out two competing hypotheses. If the tenaculum spines were denticles, they’d be skin-derived armor that just happens to look toothy.
If they were genuine teeth, they would develop and regenerate using the same cellular machinery that makes jaw teeth.
To tease this apart, the team collected hundreds of ratfish in the shallows around the University of Washington’s Friday Harbor Labs.
The specimens were scanned with micro-CT, sampled tissues for gene expression, and compared the living fish to fossil relatives.
The researchers watched the tenaculum form in both sexes.
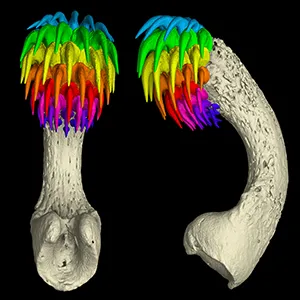
In males, a tiny cell cluster between the eyes swelled into a white bump that anchored to jaw muscles, broke through the skin, and sprouted multiple rows of sharp elements.
In females, the early cluster never mineralized, leaving only a developmental trace.
Stem cells prove tooth identity
The clearest evidence came from histology. Each row of tenaculum spines sits atop a ribbon of tissue called the dental lamina – an epithelial stem-cell niche that seeds new teeth in a conveyor belt inside the jaws of many vertebrates.
“When we saw the dental lamina for the first time, our eyes popped,” Cohen said. “It was so exciting to see this crucial structure outside the jaw.”
Dermal denticles don’t have a dental lamina. The team also found the classic “tooth toolbox” of genes active in the tenaculum but absent in skin denticles. They spotted similar head-mounted teeth in the fossil record of related chimaeras.
“We have a combination of experimental data with paleontological evidence to show how these fishes co-opted a preexisting program for manufacturing teeth to make a new device that is essential for reproduction,” said co-author Michael Coates of the University of Chicago.
Flexible teeth for reproduction
An adult male ratfish can stack seven or eight rows of these forehead teeth. Unlike our rigid canines, they retract and flex – handy when you’re trying to latch onto a mate while swimming.
The tenaculum’s growth schedule follows the maturation of the pelvic claspers, not the animal’s overall length.
This suggests that the “migrant” tooth-forming tissue is now controlled by reproductive hormonal cues and local gene networks.
Functionally, the system behaves like mouth dentitions in sharks: a renewable lamina constantly seeds replacements.
Rethinking where teeth can grow
Sharks are often the go-to model for tooth evolution because they churn out endless oral teeth and wear a coat of denticles. This study widens the lens.
The research shows that vertebrate “tooth programs” are modular and mobile. Under the right developmental signals, genuine teeth can be built well beyond the jaw margins.
Tissue samples from the tenaculum expressed the same core genetic toolkit seen across vertebrate teeth, demonstrating that these aren’t just spiky skin ornaments. “Vertebrate teeth are extremely well united by a genetic toolbox,” Cohen noted.
“If these strange chimaeras are sticking teeth on the front of their head, it makes you think about the dynamism of tooth development more generally,” said study senior author Gareth Fraser of the University of Florida.
Ancient clues from ratfish tenaculum
Finding a working dental lamina outside the mouth suggests ancient flexibility in where and why teeth evolve – whether for feeding, defense, or, in this case, reproductive grip.
The discovery also hints that evolution has repeatedly redeployed the same genetic circuitry to build similar hard parts in new places.
For paleontologists, the forehead teeth help reconcile decades-old fossils that hinted at non-oral dentitions in early jawed fishes.
For developmental biologists, they invite a new question: What other “spiky structures” might secretly be teeth?
“Chimaeras offer a rare glimpse into the past,” Cohen said. “I think the more we look at spiky structures on vertebrates, the more teeth we are going to find outside the jaw.”
In other words, the next chapter in the story of teeth may not be written in the mouth at all, but on an unassuming little hook between a ratfish’s eyes – proof that evolution’s dental toolkit travels farther than anyone imagined.
The study is published in the journal Proceedings of the National Academy of Sciences.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













