Easier method helps detect and measure the amount of plastic particles in human bodies
Scientists can pull plastic particles out of seawater with relative ease, but finding and tracking them in human body tissue is far more complex.
A new expert review lays out how labs can detect plastic particles in blood, lungs, placentas, and other tissues – and why measurement choices will shape what health risks they reveal.
The authors dissect a fast-moving literature to show where current methods succeed, where they fail, and what must improve.
They highlight the importance of sample preparation, standardized reporting, and clear metrics so that studies can be compared and results trusted.
At the center is a simple but daunting question: How do you measure plastic inside living systems without distorting the evidence?
Plastic particles in human tissue
“Every biological sample represents a different matrix,” said Baoshan Xing, professor of Environmental and Soil Chemistry at the University of Massachusetts (UMass) and lead study author.
That simple point drives the lab playbook. Fatty tissues, protein-rich organs, shells, and plant fibers need tailored digestion steps, from alkaline solutions to enzymes. These steps allow researchers separate plastics without melting or morphing them.
Researchers use the term microplastics for solid plastic particles roughly one micrometer to five millimeters in size. It’s a range now common in U.S. government and research guidance.
Nanoplastics typically refer to particles smaller than one micrometer, often defined by colloidal behavior in the one to 1,000 nanometer range.
Finding plastics in the lab
After digestion, labs isolate particles and verify their identity. The main tools are Raman spectroscopy, FTIR spectroscopy, and thermal methods like pyrolysis gas chromatography–mass spectrometry (GC/MS).
Each comes with trade-offs in size limits, throughput, and polymer specificity, especially in complex tissues. Scientists use salty solutions to float or sink plastics, making it easier to recover heavier particles.
Quality control matters as much as the chemistry, including strict contamination blanks, clean air handling, and reporting of detection limits to prevent false positives or inflated counts.
What early health signals show
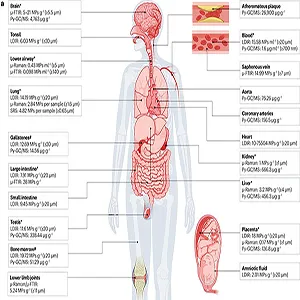
Researchers have measured microplastics in human blood, showing that polymer fragments can circulate systemically. They detected measurable mass concentrations using pyrolysis GC/MS.
Follow-up work has begun to profile sizes, shapes, and polymer types in blood from healthy volunteers to probe possible links with physiology.
Scientists have found plastic particles in human placentas on both the mother’s and baby’s sides. This raises concerns about exposure before birth, which cannot be answered without better, standardized tests.
Human lung tissue examined after surgery has contained identifiable microplastics. Micro-FTIR revealed polymer types and reported concentrations per gram of tissue under stringent blank controls.
Vascular studies have reported microplastics and nanoplastics in carotid artery plaques and associated those findings with higher risks of heart attack, stroke, or death over follow-up periods in clinical cohorts.
Methods alter outcomes
Dose matters, but researchers can count it in several ways. The number of particles per gram, mass per gram, or surface area per gram do not tell the same story in a tissue where sizes, shapes, and polymers vary widely.
Interpretation hinges on the metric. Fibers may be many but light, while spheres may be few but heavy. Different shapes behave differently, so researchers must choose measurement methods that fit the health question.
“There are no accepted protocols yet,” said Xing. Without standard methods, it’s hard to compare plastic levels across studies or turn them into health guidelines.
AI helps track plastics
High-throughput imaging and spectral data generate mountains of information, which is where machine learning (ML) can help.
Researchers are pairing Raman datasets with classification models to speed up polymer identification and reduce analyst bias.
Thermal analysis coupled with FTIR is also being automated with ML to tease apart polymer signals in complex biological matrices, improving throughput while keeping false identifications in check.
Plastics research needs standards
Reference materials, interlaboratory comparisons, and matrix specific digestion and detection protocols will make results more trustworthy and comparable across labs.
Agencies are already coordinating methods of research, from definitions to practical measurement guidance, to support that standardization push.
“This is a headache,” said Xing. The upside is that a clear, shared toolkit would let scientists quantify risks with the same confidence we expect in other areas of environmental health.
The study is published in Nature Reviews Bioengineering.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













