
Never-before-seen structure found inside a living organism has scientists baffled
Scientists have spotted a never-before-seen tubular structure inside a bacterium, now named Profftella, that lives alongside the world’s most damaging citrus pest.
The discovery opens a fresh window on how life builds complex inner machinery – and hints at new ways to fight crop-killing insects.
An international team of researchers reported this finding in npj Imaging. Their subject was Candidatus Profftella armatura, an intracellular partner of the Asian citrus psyllid, Diaphorina citri.
Profftella bacteria helps a plant pest
The psyllid is a small sap-sucking insect that devastates citrus agriculture. It harbors Profftella in its body, passing the bacterium from mother to offspring.
Profftella plays an essential role in the insect’s survival and produces toxic compounds that repel predators.
That’s why anything unusual inside this bacterium isn’t just a curiosity – it’s potentially a lever for pest control.
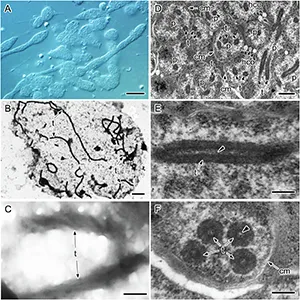
Using advanced 3D electron microscopy, the researchers found that Profftella cells – already strikingly long, sometimes over 100 micrometers – contain multiple, equally elongated tubes.
Each tube runs for tens of micrometers and measures about 230 nanometers wide. It forms from five to six right-handed helical fibers braided into a twisted cylinder.
The tubes aren’t random clutter. They keep a consistent share of the cell’s interior volume, suggesting the bacterium invests in them on purpose.
Complex organelles in bacteria
“Typically, bacteria lack such complex organelles,” said Assistant Professor Chihong Song from Pusan National University, the article’s first author.
“I was surprised to find that this tube is so stable and robust that it maintains its shape during high-vacuum electron microscopy observation.”
That durability is remarkable. Most bacterial structures collapse or need chemical treatment to survive the rigors of high-vacuum imaging. These tubes stood firm.
Peering inside the tubes
The team didn’t stop at ultrastructure. With complementary optical microscopy, they probed the tubes’ contents and found them packed with ribosomes – the tiny machines that assemble proteins.
That cargo shifts the interpretation from mere struts to something much more dynamic.
“Based on this, the tubes may be involved in protein synthesis,” said Associate Professor Atsushi Nakabachi from Toyohashi University of Technology, a corresponding author of the study.
“Given their sturdiness, they could also provide physical support to the elongated Profftella cells, and maybe even act like a scaffold for material transport – kind of like the cytoskeleton in eukaryotic cells.”
If true, Profftella would be running ribosome-rich assembly lines inside long, flexible conduits – a level of compartmentalization rarely seen in bacteria.
Bacteria aren’t so simple
Textbooks often paint bacteria as simple bags of biochemistry. That’s outdated. We’ve learned that some bacteria build internal magnets, stack membrane sacs, or parcel out enzymes.
Even so, well-defined, large, tube-like organelles are vanishingly rare.
Profftella’s structure adds a new chapter to the playbook of what prokaryotes can do, especially in the context of a tight symbiosis where the host insect’s demands may have driven the evolution of new cellular tricks.
The tubes’ repeated, helical architecture also nudges big evolutionary questions.
Did these structures arise de novo in Profftella as it adapted to life inside psyllids? Are there related elements in other insect symbionts that have been missed because they’re fragile or hard to visualize?
And do the tubes blur the line between the organizational logic of bacteria and the scaffold-rich worlds inside eukaryotic cells?
Profftella offer hope for farmers
Because Profftella passes vertically and plays a central role in the psyllid’s biology, its unique machinery could represent a soft spot.
If the tubes help the bacterium make proteins or maintain its long shape, disrupting them might undermine the symbiosis.
That could weaken the insect without broad-spectrum chemicals. It’s a tantalizing prospect for growers who face relentless pressure from citrus pests and the diseases they spread.
Any such strategy would need to be precise and thoroughly vetted for ecological safety, but clear structural targets make careful design more plausible.
Questions about Profftella remain
Open questions abound. What are the tubes made of at the molecular level? Are their helical fibers protein, RNA-protein complexes, or something entirely new?
Do the tubes connect to membranes or to the cell’s ends, and do they grow and shrink?
Most importantly, what happens to the bacterium – and to its insect host – if something blocks or dismantles the tubes?
Answering those questions will take biochemistry, genetics, and more imaging across developmental stages of both bacterium and insect.
It may also require looking beyond Profftella to see whether other symbionts in agricultural pests hide similar surprises.
For now, the take-home is simple and a bit thrilling. Inside a microscopic partner of a citrus-destroying bug, researchers have found a robust, ribosome-laden, helical tube that no one knew existed.
This challenges the way we think about bacterial simplicity, and it offers a concrete new target in the long, hard fight to protect the world’s oranges, lemons, and limes.
The full study was published in the journal npj Imaging.
from Pusan National University, Japan’s National Institute for Physiological Sciences, Kobe University, and Toyohashi University of Technology
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













