New method allows scientists to look inside an atom's nucleus
A team led by MIT used a simple molecule to peek inside a radium atom’s nucleus. In a new study, they watched electrons in radium monofluoride pick up a tiny energy change that betrays what is happening deep in the core.
The tests ran on a compact setup at CERN in Switzerland and not in a collider that stretches for miles. The result points to a practical way to map nuclear structure and to probe why the universe favors matter over antimatter.
Radium monofluoride reveals atomic secrets
Molecules can boost the internal electric fields seen by electrons, which makes rare effects easier to spot. Lead researcher Ronald Fernando Garcia Ruiz of the Massachusetts Institute of Technology guided the experiment at the Collinear Resonance Ionization Spectroscopy facility at CERN.
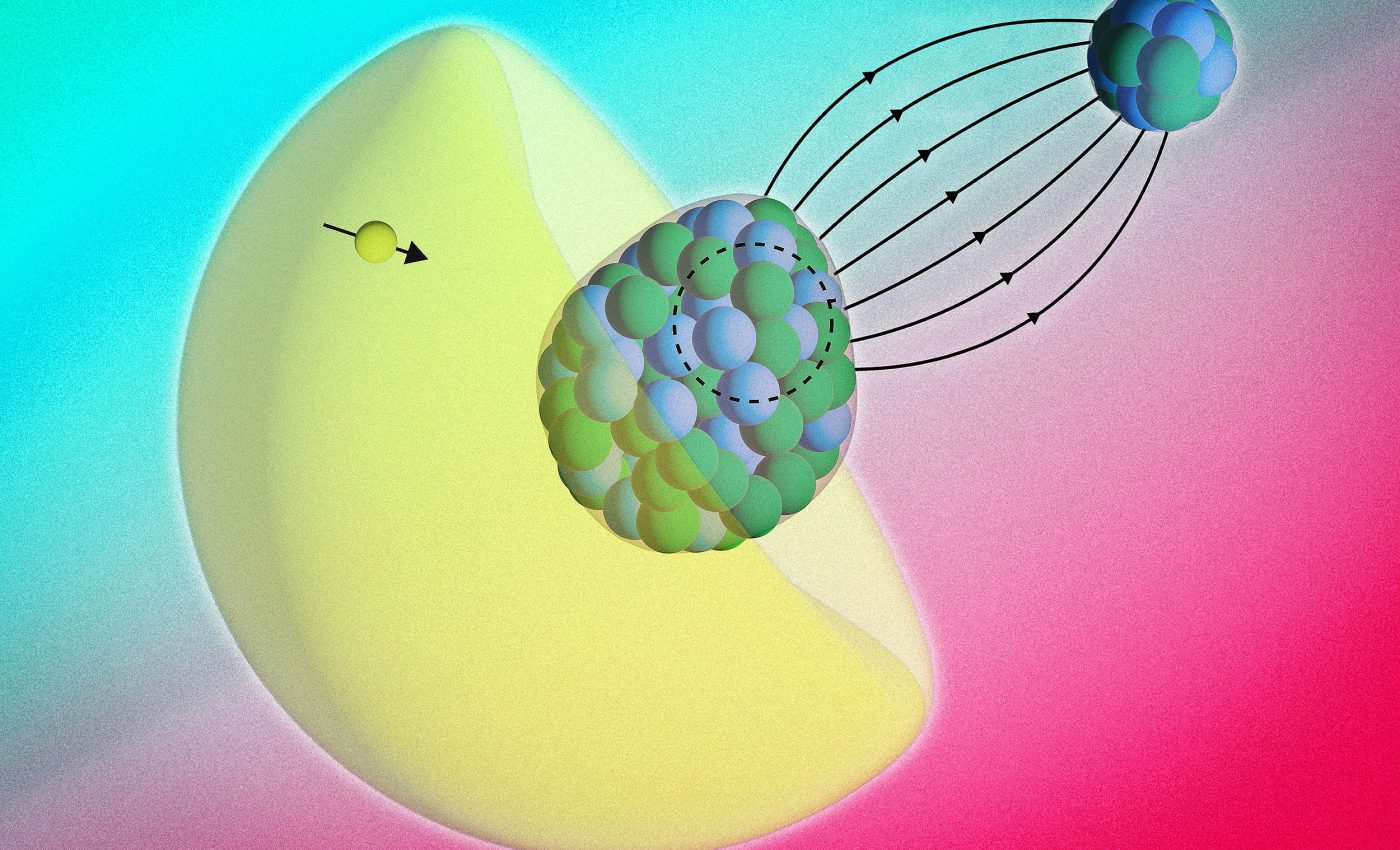
Inside radium monofluoride, the team followed shifts in the molecule’s hyperfine structure, tiny energy changes from nucleus electron interactions. These shifts act like a signature from the nucleus itself when electrons briefly pass through it.
Earlier work showed radium monofluoride is unusually sensitive to the size of the nucleus. That sensitivity is a hint that electrons in this molecule can reveal details usually hidden from view.
“We now have proof that we can sample inside the nucleus,” said Ronald Fernando Garcia Ruiz, the Thomas A. Franck Associate Professor of Physics at MIT. That is a bold claim, but here it is backed by precise measurements and careful cross checks.
Tracking electrons inside nucleus
The group paired radium with fluoride, then cooled and trapped the molecules before laser probing their electron energies. Their tally showed a small but clear offset from what theory predicts if electrons never enter the nucleus.
“When we went to measure these electron energies very precisely, it didn’t quite add up to what we expected,” said Shane Wilkins, the study’s lead author. That mismatch is exactly what you expect if electrons spend a sliver of time inside the nucleus.
The new data constrain the nuclear magnetization distribution, how magnetism is spread inside the nucleus. That property alters the hyperfine pattern in a way that advanced theory can calculate and experiments like this can test.
A 2020 analysis showed that modeling this distribution is essential for heavy systems such as radium and its molecules. The present measurements push that idea from calculation into a laboratory benchmark.
Radium atom shows rare symmetry
Radium 225 has an octupole deformed nucleus, a pear-shaped nuclear shape. That asymmetry amplifies certain symmetry breaking effects that are nearly invisible in ordinary nuclei.
Physicists care because these effects connect to time reversal and charge parity violations. Those violations might explain why matter dominates antimatter in today’s universe.
“The radium nucleus is predicted to be an amplifier of this symmetry breaking, because its nucleus is asymmetric in charge and mass, which is quite unusual,” said Ruiz. That is why the team chose radium as the heart of the molecule.
A 2024 overview from the U.S. Department of Energy describes why short lived radioactive molecules are valuable for these searches. They blend nuclear heft with laser control, a rare and useful combination.
New way to study atoms
Traditional nuclear scattering uses electron beams and detectors spread over facilities that can be several miles long. Those experiments deliver hard won images of nuclear structure, but they are costly and infrequent.
The molecule based route reads nuclear information from the light emitted or absorbed by trapped molecules. It trades brute force for precision, and it runs on a table top with lasers and vacuum chambers.
That does not make it easy. Radium is scarce and radioactive, and radium monofluoride is produced in tiny numbers that decay quickly.
Even so, the team extracted a clear signal. They saw a pattern consistent with electrons briefly sampling the nuclear interior and returning with a measurable energy shift.
What it could reveal next
With this method in hand, the next step is to map how magnetism is distributed across the radium nucleus. Doing that requires aligning the molecules and cooling them to reduce thermal motion.
Those maps would sharpen theory used to predict signals of symmetry violation. They would also help set tighter limits on quantities linked to an electric dipole moment, a tiny separation of positive and negative charges.
If future runs see hints of symmetry breaking, that would challenge parts of the Standard Model, the current theory of fundamental particles. If they do not, the null result still trims the space where new physics could hide.
Radium monofluoride opens a new view
Skeptics will ask whether stray fields or modeling choices can mimic the shift. The team addressed that by comparing multiple transitions and by using calculations that include relativistic and electron correlation effects.
It is also fair to ask whether this approach scales beyond radium monofluoride. A growing body of work now explores other heavy molecules with similar features, and several groups are developing complementary techniques.
The pay off is not just a check on theory. It is a compact way to read nuclear structure that could broaden access beyond a handful of giant labs.
The method will evolve, but the core message is simple. Molecules can carry information from inside the nucleus to a detector, and careful measurements can read it.
The study is published in Science.
Featured image: This image depicts the radium atom’s pear-shaped nucleus of protons and neutrons in the center, surrounded by a cloud of electrons (yellow), and an electron (yellow ball with arrow) that has a probability to be inside the nucleus. In the background is the spherical nucleus of a fluoride atom, which joins to form the overall molecule of radium monofluoride. Credit: Courtesy of Ronald Fernando Garcia Ruiz, Shane Wilkins, Silviu-Marian Udrescu, et al
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













