Pain relief breakthrough as scientists discover a safer, non-addictive approach
When a throbbing knee or aching back refuses to quit, many of us head straight for the pantry. A scoop of ice cream or a fistful of chips can soothe more than the soul.
Researchers have long noticed the same comfort-seeking reflex in lab animals, hinting that something in the very act of eating can turn down the volume on pain.
The phenomenon, known as ingestion analgesia, crops up even when the “meal” is a single lick of sugar water.
The idea that food might blunt discomfort has usually centered on the brain’s reward circuits.
Yet a growing body of work is pulling the spotlight toward the nerves that sit outside the skull. These peripheral messengers, it turns out, don’t just ferry pain – they can hush it, too.
Enter TRPV1 and D₂O
Scientists have discovered a novel way to relieve pain by targeting TRPV1, a key ion channel involved in sensing heat and physical discomfort.
Instead of using conventional painkillers, the approach uses heavy water (D₂O) to subtly alter the channel’s behavior, effectively reducing its overactivity.
TRPV1 is best known for triggering the burning sensation from chili peppers, but it also plays a central role in many types of pain, with the added benefit of being non-addictive.
Comfort food’s secret side effect
For rodents, a nibble of chocolate during a mild heat test leads to fewer squeaks and squirms. Scientists traced the mellowing effect to hormones that surge as digestion kicks in.
Chief among them is glucagon-like peptide-1, or GLP-1, a gut signal that rises when nutrients hit the small intestine.
In humans, GLP-1 keeps blood sugar in check by nudging the pancreas to release insulin, but its life in the bloodstream is fleeting – barely one or two minutes.
Drugmakers have stretched that clock by crafting GLP-1 analogs that linger for hours. These longer-lasting versions already help people with type 2 diabetes and, more recently, individuals seeking weight loss.
The same molecules have been shown to sap pain inside the brain and spinal cord, raising a simple, tantalizing question: could they also soothe pain at the nerve endings scattered through skin, joints, and organs?
TRPV1 channel – the gatekeeper
Fresh experiments suggest the answer is yes. GLP-1 appears to shoulder past a notorious gatekeeper of pain, the TRPV1 channel.
Think of TRPV1 as a molecular thermostat: when capsaicin from a pepper, a hot stove, or acidic conditions switches it on, ions rush through its pore, igniting a pain signal.
In chronic pain disorders, the channel can become hypersensitive, turning even a light touch into agony.
Directly blocking TRPV1 has helped in animal tests, but the strategy often wreaks havoc with body temperature, leaving patients flushed or chilled.
GLP-1 seems to take a subtler route. Instead of wedging the channel shut, it tweaks TRPV1 just enough to stop the excess firing while sparing the body’s heat control. The result is a calmer signal with no feverish side effects.
Cooling down a hot channel
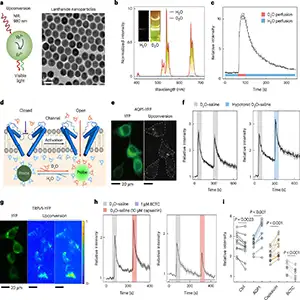
Details of that tweak came into sharper focus thanks to a collaboration that stretched from Singapore to Beijing. Professor Liu Xiaogang of the National University of Singapore and colleagues had pivoted from hormones to water – specifically, heavy water, or D₂O.
Their team fashioned a luminescent nanoprobe able to tell ordinary H₂O from its heftier cousin as the molecules darted through single TRPV1 pores.
When D₂O slipped through the channel, pain signals in nerve cells sputtered. In living models of both acute and chronic inflammatory pain, injections of D₂O eased sensitivity without muting other nerve reflexes.
TRPV1 pain management strategy
This discovery deepens our understanding of TRPV1 function and lays the groundwork for new pain-management strategies.
The research team now plans to investigate how D₂O influences additional ion channels, aiming to adapt this mechanism for treating neurological disorders and other clinical challenges.
The solvent-mediated pathway to analgesia stands out as a major advance in pain therapy and could usher in safer, non-addictive options for patients.
Although calming an overactive channel with solvent molecules may sound unconventional, D₂O is already well known in nuclear reactors and biochemical laboratories.
In small doses it poses little risk, making it an appealing complement – rather than a replacement – to existing drugs.
What happens next?
Pair GLP-1 analogs with the solvent trick and a two-pronged strategy emerges: tamp down abnormal TRPV1 activity while letting the body keep its natural thermostat.
That approach could offer relief to millions who live with neuropathic pain, osteoarthritis, or lingering post-surgical aches.
These are all conditions where current options rely heavily on opioids or non-steroidal anti-inflammatory drugs, both of which are carry-overs from the last century and are not without drawbacks.
Clinical translation will demand careful dosing studies, long-term safety checks, and creative formulations.
Yet the blueprint is simple: use a compound we already make after every meal, fortify it so it sticks around, and harness its knack for silencing wayward pain channels.
Add a dash of heavy water for extra finesse, and the humble act of eating might inspire the next generation of pain relief – no prescription for comfort food required.
The full study was published in the journal Nature Biomedical Engineering.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













