Stunning images show exactly how antibiotics destroy bacteria
Antibiotics don’t always take out bacteria in one clean hit; sometimes they require a little push to jump-start them, as was discovered in a recent study after researchers added a variety of sugars to strands of E. coli.
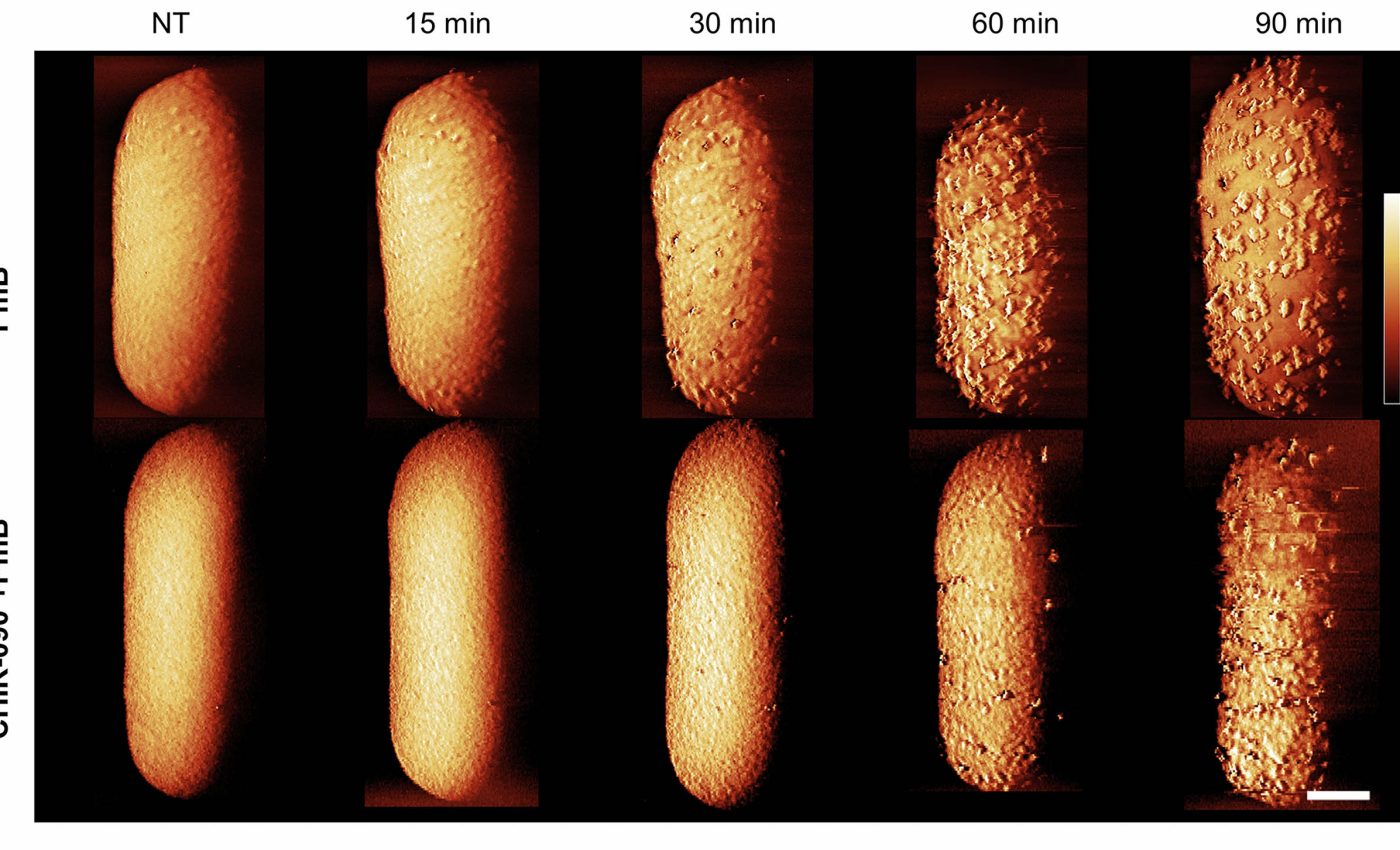
The new set of images shows step-by-step how the powerful peptide antibiotic, polymyxin B, tears open E. coli’s protective surface, then slips inside to finish the job. The catch is striking.
The antibiotic only succeeds when the cells are active, and it stalls when cells are in a low-energy, dormant state that often pops up inside the body.
Antibiotic evades E. coli defenses
The research was led by Andrew M. Edwards of Imperial College London. The study revealed what happened when polymyxin B latched onto E. coli and pushed through its defenses.
The bacterium belongs to the gram-negative group, which carries an extra outer layer rich in lipopolysaccharide (LPS) that acts as a shield.
The team used atomic force microscopy (AFM) to watch that shield change shape in real time as the drug went to work.
Watching antibiotics kill E. coli
Within minutes, the drug sparked small surface bulges on the outer membrane, followed by fast shedding of lipopolysaccharide.
Those changes opened short-lived gaps that let the antibiotic reach the inner membrane and disrupt it. However, metabolic activity plays a crucial role in the effectiveness of polymyxin B.
The antibiotic readily eliminated actively growing E. coli, but cells in a dormant phase surprisingly survived until they were supplied with a carbon source.
The study definitively showed why energy matters. When metabolic activity is high, the cells keep creating and moving lipopolysaccharide to its surface, and the drug rides that churn to punch holes.
When cells are dormant, that traffic slows to a crawl, and the drug stalls at the surface.
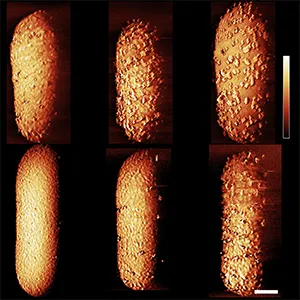
After applying a variety of sugars to the dormant bacteria, the team witnessed approximately a 15-minute delay from the application process to the killing of the E. coli; the time needed to restart the cell’s armor assembly line
How resistance can spread
In 2015, a landmark study reported MCR-1, a mobile resistance gene that tweaks lipid A with phosphoethanolamine and lowers polymyxin binding.
That finding explained how resistance can spread quickly between strains. The new work helps connect that chemistry to what we see on the cell surface.
When MCR-1 is present, the drug still grabs the outer membrane, but the dramatic lipopolysaccharide loss does not kick in, so access to the inner membrane is blocked.
Sugar is not the defining solution
Researchers found that feeding cells sugar woke dormant bacteria, then the antibiotic completed the kill after that brief delay.
That sounds like a tempting add-on, but it can be a risk for patients because reviving pathogens can worsen disease.
A safer idea is to pair treatments so the drug reaches its target without waking the cells. The study hints at combinations that prime the outer membrane, then let polymyxin B cross and hit the inner membrane.
The top three pathogens on the latest WHO priority list are gram-negative bacteria that often resist multiple drugs. Polymyxins remain a fallback, so understanding their limits is not an academic exercise.
Hospitals consistently see stubborn infections that wax and wane. This mechanism, where dormancy shields cells from a last-line drug, offers a concrete reason and a path to test smarter combinations.
High-tech imaging breakthrough
Atomic force microscopy is a scanning method that drags an ultrafine tip over a cell and feels its contours.
This technology allowed the team to follow the same bacterium over time and catch the moment when protrusions formed on the surface.
Those protrusions rose while lipopolysaccharide levels in the medium climbed, linking shape changes to armor shedding.
The images did not show the protein network of the outer membrane collapsing, which helps explain why cells can shed a lot of LPS and still hang on until the inner membrane is breached.
Tolerance is not the same as resistance
The stalled killing in dormant E. coli cells is a form of tolerance, not a genetic change that blocks the drug. It depends on the state, not a mutation, and it can reverse when nutrients return.
This distinction matters for care. Tests that read out susceptibility in rich media can miss this energy link, so dosing plans built only on those numbers may overpromise in real infections where nutrients are scarce.
Antibiotics, E. coli, and future study
“For decades, we’ve assumed that antibiotics that target bacterial armor were able to kill the microbes in any state, whether they’re actively replicating or they were dormant,” said Edwards.
That assumption does not hold here, and the images make the weak point visible. The drug needs the cell’s own LPS traffic to open its way in.
Two paths stand out. One is to identify non-nutritive helpers that loosen the outer membrane so polymyxin B can reach the inner membrane without switching on growth.
The other is to tailor timing, dose, and combinations to the metabolic state of the infection site. That means pairing pharmacology with better state-aware diagnostics, not just culture results from a lab broth.
The study is published in the journal Nature Microbiology.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













