Quick and easy way to extract valuable materials from old tech devices
Old phones, spent fluorescent bulbs, and other cast off gadgets pile up worldwide, even though many of them contain small but precious amounts of rare earth elements such as europium. In the European Union, less than 1 percent of these metals are recycled each year.
Meanwhile China refines about 90 percent of the global supply of processed rare earths, giving it enormous leverage over clean energy supply chains
A new study from ETH Zurich has introduced a fast and efficient way to recover the rare earth metal europium from old electronic waste, using a simple sulfur-based compound.
The research highlights a low-cost and environmentally friendly recycling method that could significantly improve how rare earth elements are recovered from discarded fluorescent lamps and other devices.
Europium in old gadgets
The idea comes from Marie Perrin, a doctoral chemist at ETH Zurich, who discovered a shortcut for prying europium out of discarded fluorescent lamps.
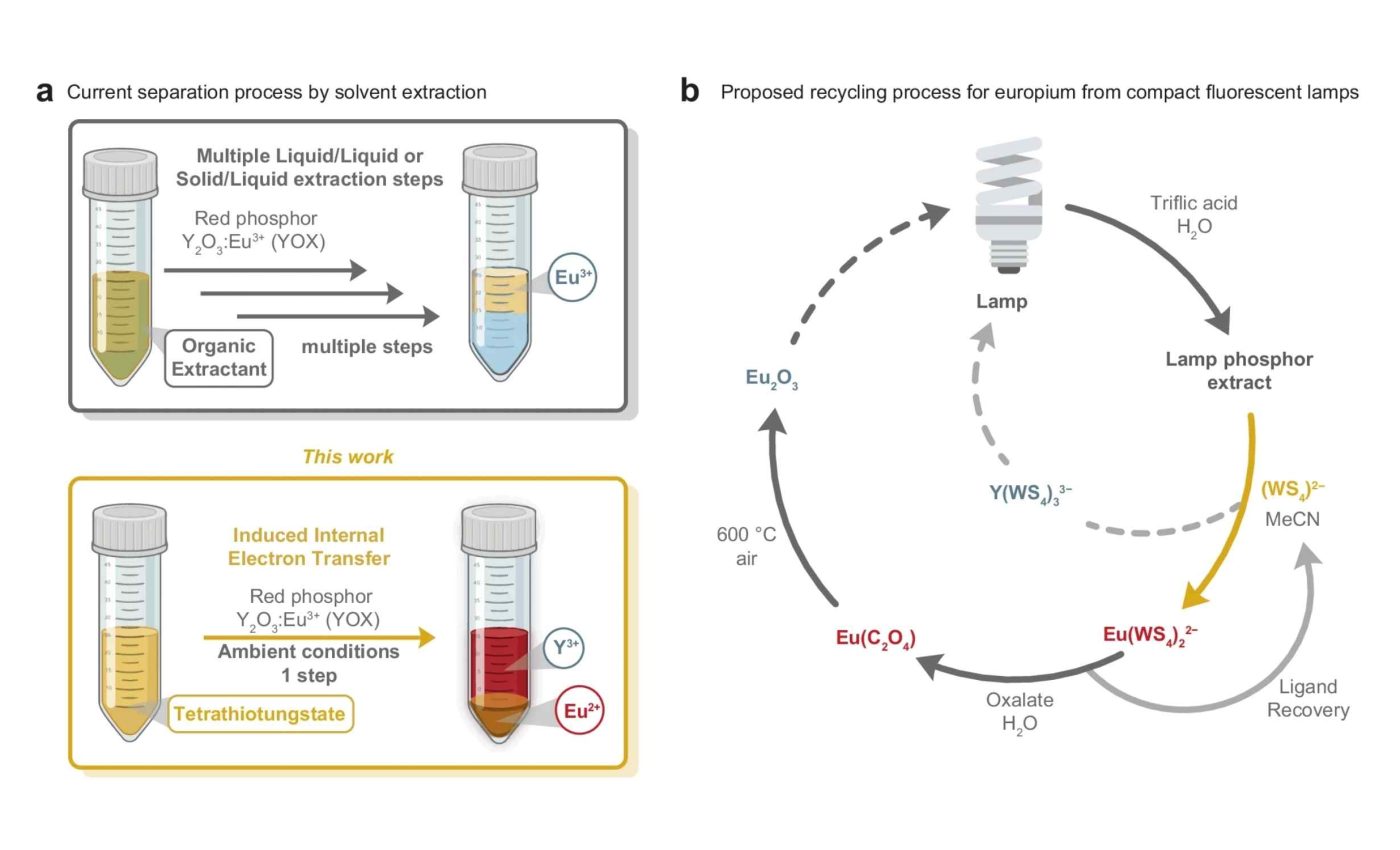
Her tweak replaces the hundred plus solvent extractions usually needed for the job with one quick reaction.
“Rare earth metals are hardly ever recycled in Europe,” noted Victor Mougel, professor of inorganic chemistry at ETH Zurich.
That scarcity of recycling pushed his group to find a method that can run in an ordinary lab flask instead of a chemical factory.
Most bulbs that light Swiss basements still contain phosphor powder whose rare earth content is about 17 times richer than the best natural ores.
Recovering those metals locally would turn what is now shipped abroad for disposal into a home grown resource.
When the researchers tested their system on crushed lamps, they pulled out nearly all the europium in a single pass. They did it without first roasting or acid leaching the powder that usually gums up recycling plants.
How sulfur atoms come to the rescue
Perrin’s secret weapon is a yellow powder made of sulfur and tungsten that works like a metal magnet. It gives europium a little electrical nudge that changes its charge, making it easier to pull out of the mix.
That little flip matters because the divalent metal does not like to stay dissolved, so it crashes out of solution as a solid coordination polymer. The precipitate can then be scooped off with a filter in minutes.
“This allows us to obtain europium in a few simple steps and in quantities that are at least 50 times higher than with previous separation methods,” explained Marie Perrin, the study’s lead author. The reaction works under room light or with mild heating instead of ultraviolet lamps.
What makes this powder work so well is how its sulfur parts shift electrons around inside the molecule.
One part gives up an electron, while another part passes one along to europium, helping it change form. This clever handoff replaces the need for strong and harmful chemicals usually used in the process.
Skipping the chemical obstacle course
Traditional rare earth refineries rely on a chain of liquid extractions that can number well over 100 stages. Each stage uses acids, kerosene, and electricity, driving both cost and emissions.
In comparison, the ETH Zurich method pulls europium out over a thousand times better than similar metals like yttrium, all in one simple step.
It works far better than current industry methods and leaves behind almost no europium in the leftover mix.
Cutting process steps slashes energy use and shrinks the footprint of future recycling plants. It also helps that the only specialty reagent, tetrathiometallate, can be washed free and reused.
Because the only by-product is a small amount of oxalate, the team calculates that the new process cuts greenhouse gas emissions by more than 80 percent compared with ore mining. That reduction matters to manufacturers facing tighter carbon accounting.
Electronic trash turns local treasure
Europe discards millions of compact fluorescent lamps each year even though their sales have fallen sharply. Sending the powder abroad for landfill wastes the phosphors and punts the environmental cost elsewhere.
In Switzerland alone, the lamp waste now marked for export could supply all domestic demand for europium if it were processed onshore.
Local recovery would also sidestep the geopolitical risks tied to China’s near total dominance of refined rare earths.
Globally, the mountain of electronic trash reached 62 million metric tons in 2022, and it is on track to top 82 million by 2030.
Shaving just a slice of the valuable metals out of that torrent could pay for cleaner disposal of everything else.
Perrin and Mougel have already started a spin off called REEcover to move the process from bench beakers to pilot scale.
Their first goal is a compact unit that handles worn out bulbs, but they are eyeing magnets rich in neodymium and dysprosium next.
Closing the loop on chemicals
After the europium drops out, engineers dissolve it briefly in water and oxalate to strip the sulfur complex. Heating that slurry turns it into europium oxide ready for new phosphors or specialty magnets.
Meanwhile the sulfur tungsten ligand regains its yellow color and can be recycled almost indefinitely. Keeping both metal and reagent in circulation fits neatly into the circular economy targets that regulators are drafting across Europe.
Bench tests show the ligand loses less than 5 percent activity after ten cycles, and the spent solution contains trace tungsten that can be recovered by simple crystallization.
That durability answers one of the biggest criticisms of reagent based recycling schemes.
What happens next
If the chemistry can be tuned for those other metals, it could shore up supplies essential for wind turbines and electric vehicle motors. The International Energy Agency projects that rare earth demand could rise three to sevenfold by 2040.
Scaling up will require engineering work on solvent handling and ligand recycling, but the basic reaction has already passed an economic reality check at lab scale.
No expensive inert atmosphere was needed and the reagent is stable enough to ship as a dry powder.
Investors have noticed the simplicity, and the team expects to raise seed funding once patents clear. If they succeed, the yellow powder from an academic lab may soon show up in the back rooms of recycling centers around the world.
The study is published in Nature Communications.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













