Scientists discover a 'mortality timer' inside human cells
Cells do a lot of work to stay alive, and much of that work depends on how they organize their interiors. One small compartment inside the nucleus has come into focus with regards to aging: the nucleolus.
It resembles a liquid droplet and lacks a membrane, yet it maintains a clear boundary that separates it from the rest of the nucleus. Its main job is simple and central to life: building ribosomes so the cell can make proteins.
Research at Weill Cornell Medicine asked a direct question that goes to the core of aging biology: does the size of the nucleolus act like a timer that predicts – and even contributes to – the end of a cell’s life?
Their study focuses on how nucleolar size connects to genome stability, especially in a fragile stretch of DNA that is repeated many times.
Understanding the nucleolus
The nucleolus serves as a factory for the early steps of ribosome assembly. Many textbooks label it a “workshop” because it brings together the RNAs and proteins that will become ribosomes.
This compartment keeps an orderly boundary, allowing the right molecules in and keeping others out.
Tucked into the nucleolus is ribosomal DNA (rDNA). Unlike single-copy genes, rDNA is organized as long arrays of repeated sequences. That arrangement lets cells produce large amounts of ribosomal RNA quickly.
Repeated sequences come with a cost. When a cell copies or repairs DNA, similar repeats can misalign, leading to deletions, duplications, or rearrangements.
Those mistakes in the nucleolus destabilize the genome, and genome instability is a known feature of aging.
“Aging is the highest risk factor for these diseases,” said Dr. Jessica Tyler, professor of pathology and laboratory medicine at Weill Cornell Medicine.
“Rather than treating each disease separately, a better approach would be to develop a therapeutic or supplement that will delay the onset of diseases by preventing the underlying molecular defects that cause them.”
The nucleolus may hold the key to combat aging at the cellular level.
Nucleolus size and aging
Researchers have long noted that nucleoli tend to grow in older cells, while smaller nucleoli show up in some long-lived cells or in cells exposed to certain longevity interventions.
Correlation alone does not settle cause and effect. The study’s goal is to move from “these things go together” to “this thing is actively driving that outcome.”
The question is whether nucleolar size itself pushes cells toward the end of their lifespan by changing the way rDNA is protected or exposed.
Budding yeast offers a clean way to test cellular aging. In this organism, a “mother” cell divides a finite number of times and then stops, defining its replicative lifespan.
Counting divisions gives a direct readout of how long a single cell stays productive. That simple metric supports experiments that ask whether a specific change, like altering nucleolar size, nudges lifespan up or down.
Yeast in the lab
Dr. Tyler and postdoctoral fellow Dr. J. Ignacio Gutierrez, the first author of the paper, hypothesized that keeping a nucleolus small could delay aging.
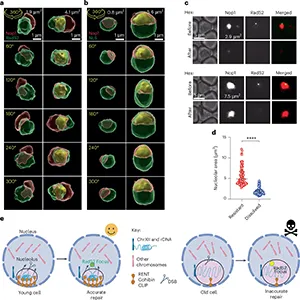
To test this idea, The team engineered yeast so the nucleolus stayed smaller than usual. They followed single cells over time and counted divisions.
“The advantage of our system is that we could isolate the nucleolus size from all of the other effects of anti-aging strategies,” Dr. Gutierrez said.
Cells with smaller nucleoli completed more rounds of division before stopping. The intervention extended replicative lifespan, pointing to a protective effect of compact nucleoli.
When big becomes too risky
Aging cells often show an enlarged nucleolus, but the effect here was not a smooth “bigger equals worse” pattern. Evidence pointed to a critical threshold.
Below that point, the nucleolus acted like a selective droplet with a firm boundary. Above that threshold, the material properties shifted. The boundary lost tight control, changing what could cross in and out.
After hitting this threshold, cells survived for an average of just five more cell divisions.
Selectivity matters because it controls which proteins can contact rDNA. In a healthy nucleus, only a defined set of molecules gains access to that repeated DNA.
Once the nucleolus swelled beyond the threshold, proteins that usually operate elsewhere slipped inside. The boundary became more permeable, and rDNA lost its selective protection.
Anti-aging by targeting nucleolus
A straightforward explanation would claim that smaller nucleoli produce fewer ribosomes, slow growth, and incidentally lengthen lifespan. The data argue otherwise.
Lifespan extension did not track with bulk ribosome output or with general silencing of rDNA. The measure that lined up with added lifespan at the cellular level was rDNA stability.
Cells with smaller nucleoli showed fewer harmful rearrangements across the rDNA array. A compact nucleolus appears to shield rDNA so the genome stays organized for longer. If it swells beyond a threshold, protection weakens, and it dies.
“When we saw it wasn’t a linear size increase, we knew something really important was happening,” said Dr. Gutierrez. Passing the threshold appears to serve as a mortality timer, ticking down the final moments of a cell’s life.
During the next phase, researchers can probe this by tracking nucleolar size alongside markers of DNA repair using human stem cells.
If the pattern holds, nucleolus size control could join a short list of factors that influence aging and how long human cells remain functional.
The full study was published in the journal Nature Aging.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













