Scientists manage to slow the course of Huntington's disease for the first time
For the first time, doctors have watched a treatment slow the course of Huntington’s disease in people, instead of just easing symptoms. In a small international trial, people who received an experimental, one-time treatment called AMT 130 declined about 75 percent more slowly than similar patients who did not.
One participant who had to stop working because of symptoms has even been able to return to a job, several years after treatment commenced.
For families who have only known this disease as a slow and unstoppable slide, that kind of stability feels like a very different future.
Huntington’s disease progression
This work is being led by Prof. Sarah Tabrizi, a clinical neurologist at University College London. She has spent many years caring for and studying people with Huntington’s disease.
Her research zeroes in on the earliest changes in the disease’s progression, and on ways to slow damage long before people lose their independence.
Huntington’s disease is a hereditary neurodegenerative condition that slowly destroys brain cells. A faulty HTT gene drives the process.
Because the mutation follows an autosomal dominant pattern, each child of an affected parent has a 50 percent chance of inheriting it. Symptoms eventually develop in mid-adulthood.
Once movement problems, thinking changes, and mood symptoms appear, the illness usually worsens over about two decades. This often leaving people completely dependent on outside care.
Until now, available medicines have treated chorea, irritability, or depression, but none have reliably slowed the underlying disease process.
Gene therapy and AMT 130
The basic idea behind gene therapy is to give cells new genetic instructions so they make less of a harmful molecule or more of a helpful one.
In conditions like Huntington’s, that means turning down the production of a toxic protein instead of just patching over the damage it causes.
The treatment uses an engineered viral vector that carries custom DNA into neurons. Inside those cells, the added DNA drives production of a short RNA molecule that locks onto the huntingtin message. The protein is then flagged for destruction so that less mutant protein is made.
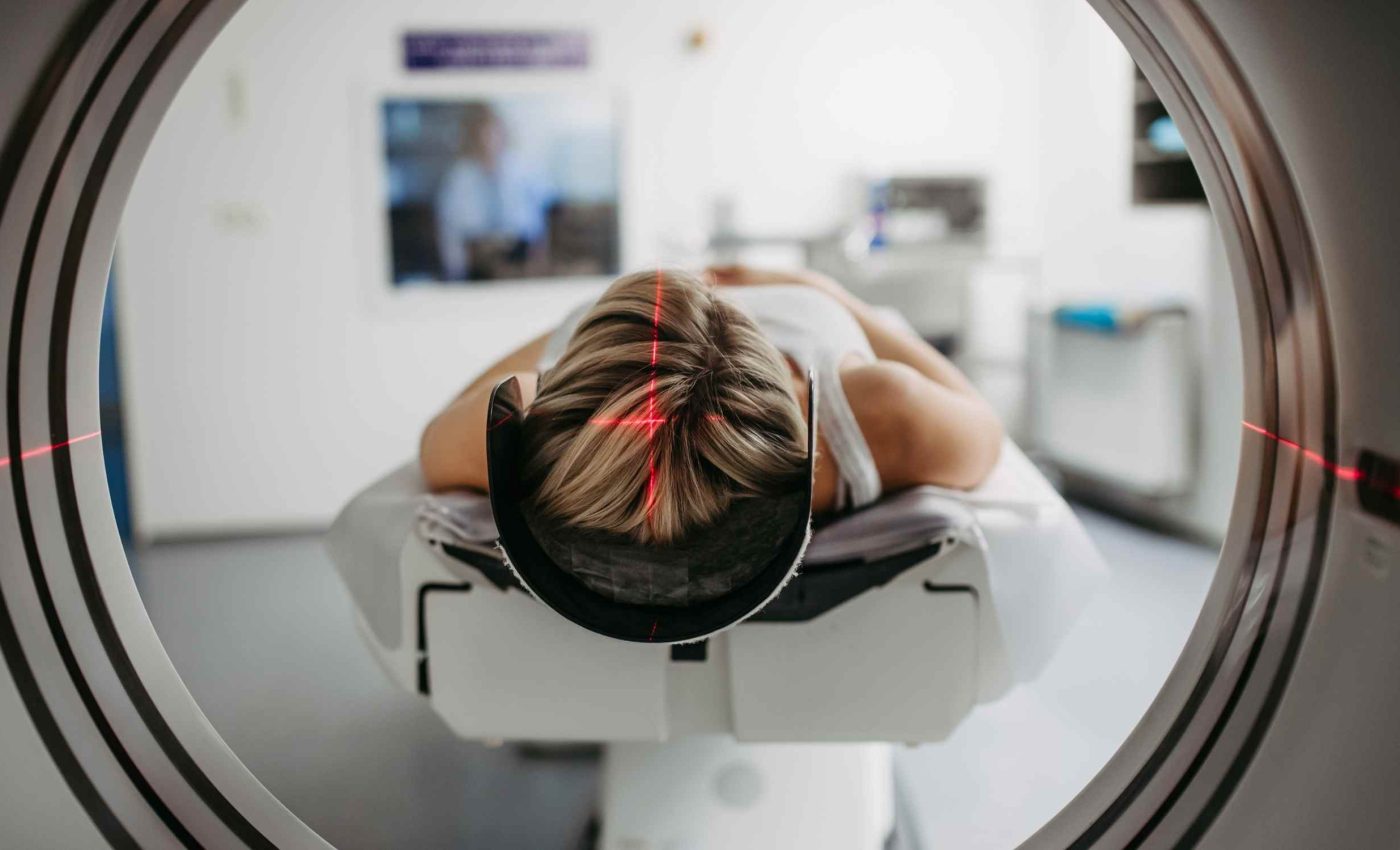
Doctors deliver AMT 130 in a single, very long operation. They use precise, stereotactic neurosurgery to infuse the virus into deep brain regions within the striatum.
The surgeons use stereotactic techniques to guide instruments with three-dimensional imaging. This helps them thread thin catheters into exactly the right spot while the person is in an MRI scanner.
Inside the AMT 130 trial
In this initial phase study, scientists gave twenty-nine volunteers with early Huntington’s symptoms either a low dose or a high dose of AMT 130. They followed all volunteers for three years after surgery.
To judge whether the treatment helped, researchers compared treated volunteers to data from Enroll-HD. This is a long-running observational study that tracks more than twenty thousand people from Huntington’s families around the world.
On an overall rating scale that blends movement, thinking, and daily functioning, the high dose group showed about a 75 percent slower decline than the matched control group over those three years.
On a separate scale that measures independence in day-to-day tasks, the same group retained about 60 percent more function than would normally be expected at that stage.
Trial results come through in numbers and graphs. However, each datapoint represents a patient who volunteered to undergo major neurosurgery and receive the first gene therapy ever tested in Huntington’s disease.
What the falling biomarker signals
Besides watching symptoms, the team tracked levels of neurofilament light chain. This is a structural protein that leaks out when long nerve fibers are injured.
Higher levels of this protein in blood or spinal fluid usually mean faster ongoing damage in many brain diseases, including Huntington’s.
In the high dose AMT 130 group, average neurofilament light levels in spinal fluid actually fell by about 8 percent during the follow up instead of rising as expected.
That drop fits with the relatively stable clinical scores and suggests that the therapy is slowing the constant drip of neuronal injury rather than simply masking symptoms.
Serious side effects have mostly related to the brain surgery itself, such as headaches and temporary swelling near the infusion sites, and have generally eased over time.
Across dose groups, safety reports so far describe AMT 130 as well tolerated overall, with a safety profile that regulators can realistically work with in future studies.
Hurdles between trial and everyday care
The surgery for AMT 130 takes many hours in a highly specialized operating room and requires teams that are comfortable placing catheters deep into the brain while watching MRI scans in real time.
Only top neurosurgical centers offer that kind of setup, so even if the treatment is approved, scaling it up fairly across countries will not be simple.
Even with the impressive clinical and biomarker signals, regulators still need more evidence before this approach can move from research into standard medical practice.
Next steps will likely include larger randomized studies, efforts to refine who benefits most, and discussions about how to combine this strategy with other huntingtin-lowering approaches that are already in development.
Details of the trial were published in the European Medical Journal.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













