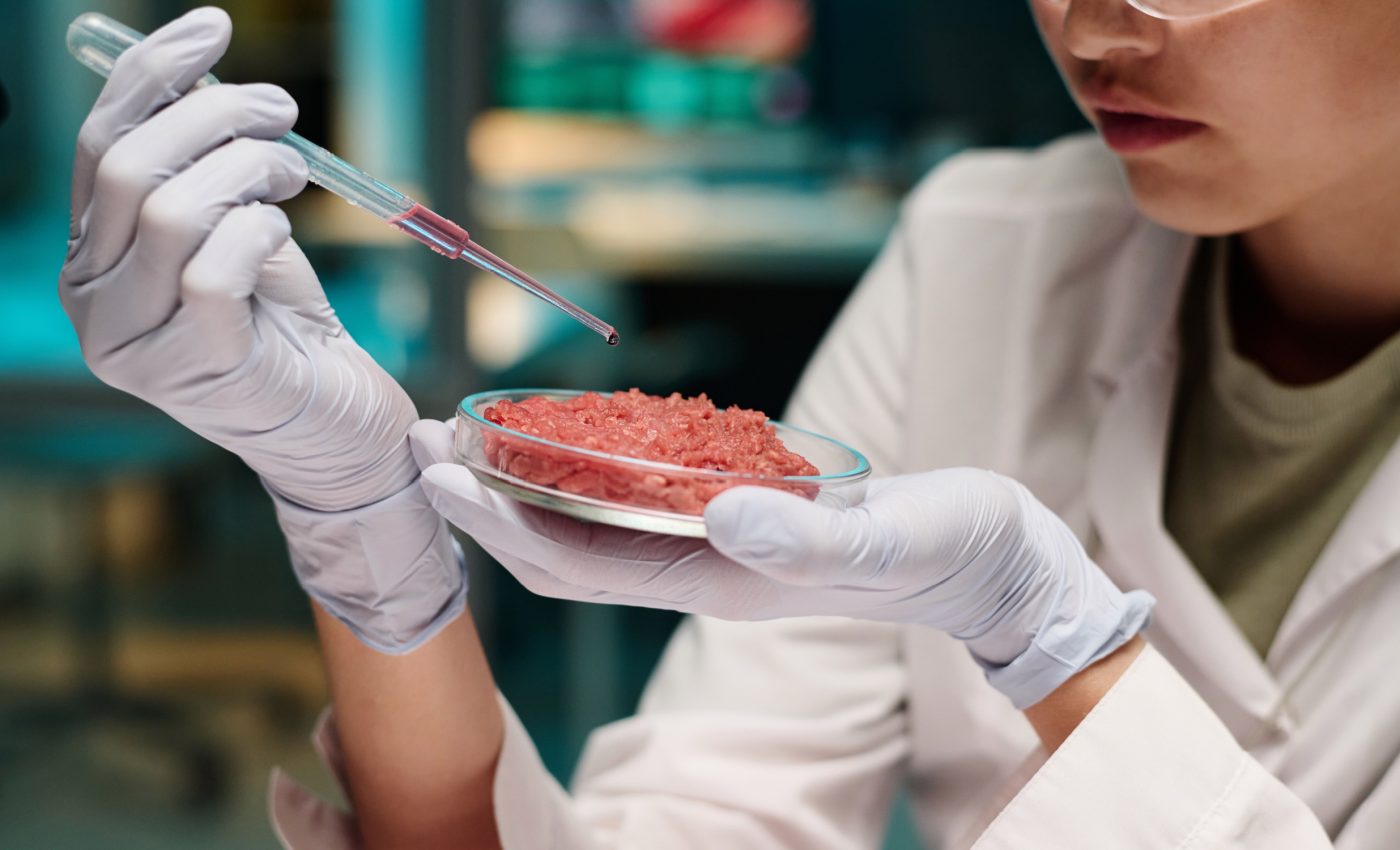
Burgers without the cow? Lab-grown beef is closer to reality
In a lab at ETH Zurich, muscle biologist Ori Bar-Nur is growing beef without the cow. His team cultivates meat from bovine cells using advanced tissue engineering techniques – but regulatory approval is still needed before anyone can take a bite.
While Bar-Nur hasn’t sampled it himself, some of his colleagues have taken part in official tastings of lab-grown beef under approved conditions. They describe its taste and texture as similar to real meat. After all, it is beef.
Instead of relying on entire animals, Bar-Nur’s team starts with a small biopsy from a living cow, isolating precursor cells called myoblasts.
These cells, which naturally develop into muscle fibers, are cultured in the lab to grow tissue from familiar cuts like filet, sirloin, cheek, and flank.
Turning cells into steak
Scientists had previously managed to generate muscle fibers from bovine myoblasts in the lab, but these fibers were thin. The ETH team has now created three-dimensional muscle tissue with thick fibers from myoblasts.
This tissue more closely resembles natural bovine muscle tissue both molecularly and functionally. It contains the same active genes and proteins as natural muscle and contracts in the same way.
Older methods failed to produce such qualities because the lab-grown cells lacked some key proteins. This advancement builds on a better understanding of myogenesis, the process by which muscle precursor cells differentiate into muscle fibers.
In natural systems, satellite cells activate, become myoblasts, and fuse into myotubes, a process regulated by transcription factors such as MyoD and Myogenin.
Conventional methods often used high-serum growth conditions followed by serum reduction, which led to a loss of myogenic potential and heterogeneous cell populations.
The ETH team improved on this by applying a refined medium and using molecules that support both progenitor maintenance and robust differentiation.
Lab meat is spreading worldwide
Bar-Nur’s research could influence a field poised to change global meat production. Dozens of start-ups worldwide are racing to make lab-grown meat affordable, reducing the need for cow sheds, livestock transport, and slaughterhouses.
This production method also requires less land. Although its climate impact is still debated, many believe it could be more sustainable.
Singapore has already approved commercially available lab-grown chicken produced from cultured animal cells. Lab-grown beef, however, is still in development. The recent ETH advances could push progress forward in this area.
New recipe for lab meat
To create thick, functional muscle fibers, ETH Zurich researchers added a combination of three molecules to the nutrient-rich cell culture medium. These molecules drive cell differentiation, enabling better muscle formation.
Bar-Nur developed this cocktail seven years ago during his postdoctoral work at Harvard University. The same combination – Forskolin, RepSox, and CHIR99021 – was shown in recent studies to encourage the formation of larger, more contractile muscle fibers while maintaining a pool of progenitor-like cells.
At the time, he was working primarily with mice to study muscle cells for treating hereditary muscle diseases.
Research into muscular dystrophy remains a central focus for Bar-Nur at ETH Zurich. He later discovered that the same method works well for creating higher-quality bovine muscle cells.
Transcriptomic and proteomic studies further confirmed that these improved conditions increase the expression of muscle-specific genes and proteins linked to contraction and metabolic maturity. They also promote fibre diversity, mimicking slow- and fast-twitch muscle types found in natural beef.
Making lab beef ready for everyone
The three molecules are necessary only in the early stages of muscle fiber development. They can be removed later, ensuring that any future commercial products will not contain them. Still, more work is needed before lab-grown beef reaches consumers.
“The cell culture medium requires further optimization to make it more affordable and safe for consumption,” said Christine Trautmann, a doctoral student and a lead author of the study. “Additionally, we need to explore ways to produce these muscle fibers in larger quantities.”
At present, the team has only produced a few grams of muscle and is now exploring scale-up strategies. Enhanced media and advanced 3D scaffold technologies could be critical in achieving this, as they allow tissue constructs to form with improved organization and nutritional relevance.
“These innovative new food products will have to undergo a prolonged and complex authorization procedure before they reach shop shelves and, ultimately, our plates,” said Adhideb Ghosh, the other lead author in Bar-Nur’s group.
To advance this technology and bring it to market, Bar-Nur is considering launching a start-up. His goal is to make ethically sound burgers that are both safe and affordable.
The study is published in the journal Advanced Science.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













