Cutting-edge nanotechnology reverses Alzheimer's disease in new study
An international team used nanotech to restore the brain’s cleanup barrier in mice with Alzheimer’s symptoms. This approach cut toxic amyloid beta, a protein that can clump and harm neurons, by about 45 percent within hours.
The work comes from researchers at the Institute for Bioengineering of Catalonia and West China Hospital, with collaborators in the United Kingdom.
Nanotech, the brain, and Alzheimer’s
The blood-brain barrier, a dense capillary interface that shields brain tissue and manages transport, is central to brain health.
It falters in those with Alzheimer’s disease, and studies consistently find increased leakiness and disrupted transport in sufferers.
A key player is LRP1, a receptor that helps move amyloid out across the barrier. Deleting LRP1 only in brain vessels reduces amyloid efflux and impairs memory in mice.
The work was led by Giuseppe Battaglia, ICREA Research Professor, at the Institute for Bioengineering of Catalonia (IBEC). His research centers on nanoscale materials that control traffic at this barrier.
Another process at work here is transcytosis, the shuttling of cargo through endothelial cells. Work in models maps a PACSIN2-guided route that supports amyloid clearance across this barrier.
Nanocarriers and receptors
The team built polymersomes, tiny hollow polymer spheres with programmable surfaces. The nanocarriers were tuned to bind LRP1 with mid-strength that favors productive transport.
By nudging receptors toward tubular carriers, the constructs act as both treatment and catalyst. This strategy aims to repair a faulty clearance system rather than merely deliver a cargo.
“The long-term effect comes from restoring the brain‘s vasculature. We think it works like a cascade: when toxic species such as amyloid-beta accumulate, disease progresses,” said Battaglia. He added that once the vasculature functions again, harmful molecules are cleared and balance returns.
This approach treats the barrier as injured tissue that can recover. That perspective widens the search for disease-modifying therapies.
Results from the mouse test
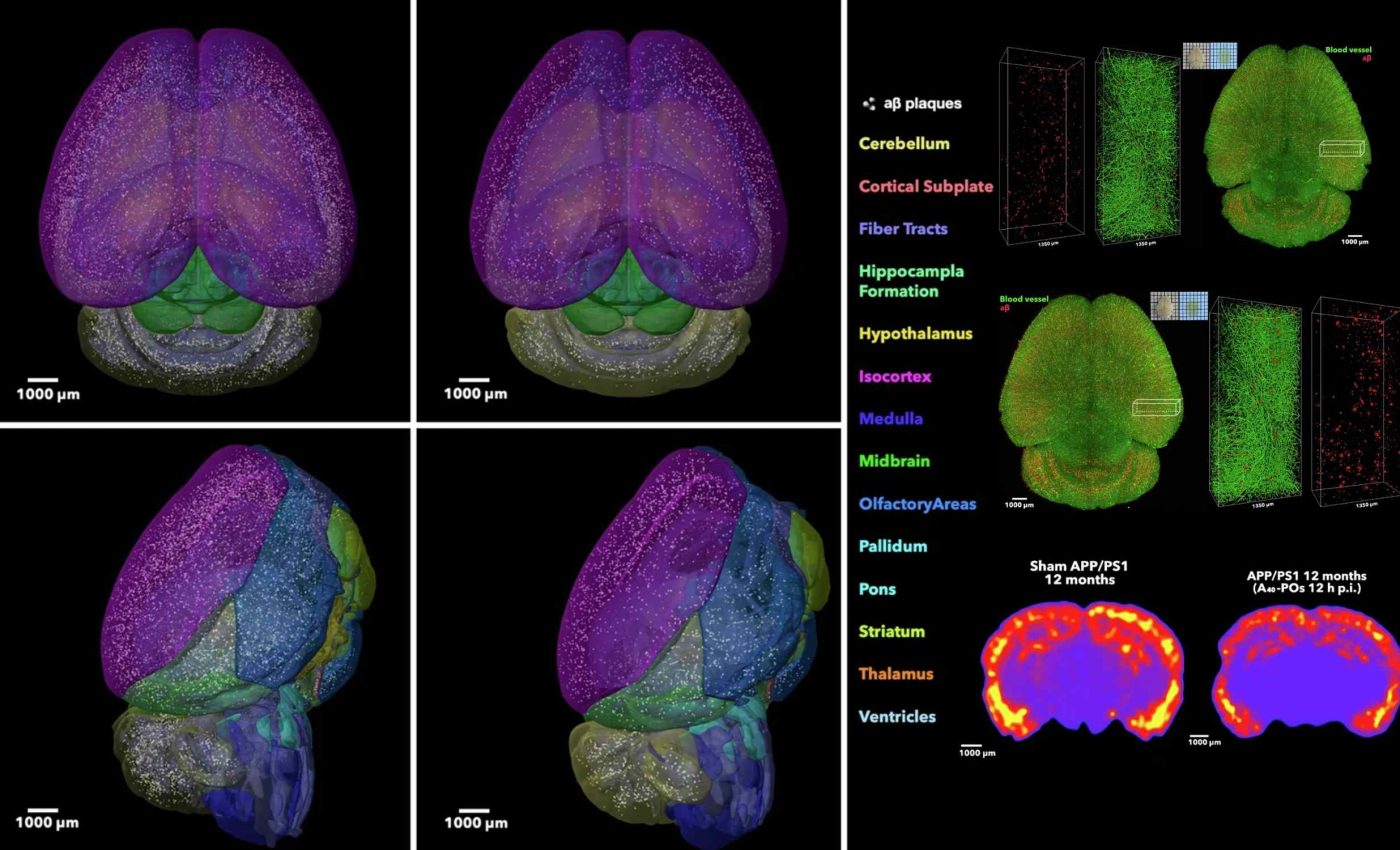
In the team’s paper, the nanocarriers lowered brain amyloid by nearly 45 percent within 2 hours. At the same time, plasma amyloid rose about eight fold, which is consistent with restored efflux across the barrier.
In water-maze tasks, treated mice matched healthy peers on learning and memory for up to 6 months. Those gains suggest the biology changed and the results were not simply a short-lived mask of symptoms.
The animals were monitored across disease stages to gauge durability. Improvements held even in older mice, a crucial hurdle in typical preclinical work.
No unusual motor differences appeared that would confound cognitive readouts. That detail matters because slower swimming can distort maze results.
Changes inside mouse brain vessels
In mice with Alzheimer’s pathology, markers of vessel function moved toward a healthier pattern. The barrier’s transporter profile shifted with more PACSIN2 – a membrane scaffold that stabilizes tubular carriers – and less Rab5-linked degradation.
Imaging showed fewer deposits hugging vessel walls and a stronger signal in the vascular lumen after dosing. That pattern fits a system that is pulling waste back into circulation.
The LRP1 signal regained its overlap with endothelial markers. Together these changes indicate a barrier that is working again, and is not just temporarily forced open.
Such vascular restoration aligns with the broader idea that damaged clearance can drive disease. Fix the clearance, and proteotoxic stress can relax.
Nanotech, Alzheimer’s, and the future
Few treatments target the barrier’s transport machinery directly. Most try to cross it, not repair it. This study reframes design goals toward rebalancing receptor traffic.
Mid-strength engagement can steer receptors away from degradation and back into cycling.
That shift could complement Alzheimer’s therapies that target other proteins. It could also help explain why some high-affinity strategies falter at the barrier.
Biophysics matters here, from ligand spacing to avidity. Those knobs control how receptor clusters move inside cells.
These results were recorded in mice; mouse models capture only parts of human disease. Human barrier biology varies with age and genetics, and that variation will shape dosing, safety, and benefit.
Receptor levels can differ across people and over time. Manufacturing and reproducibility must also meet clinical standards. The next steps include toxicology, pharmacokinetics, and human-relevant models.
Only careful trials will show whether the same repair happens in people.
The study is published in Signal Transduction and Targeted Therapy.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













