Previously unknown properties discovered in a metal that is crucial to modern life
Nearly every modern gadget relies on metals that behave exactly as textbooks predict. Gallium refuses to play along. Nearly 150 years after French chemist Paul-Émile Lecoq de Boisbaudran added the element to the periodic table, researchers are still sorting out why this silvery liquid shifts its personality when the heat is on.
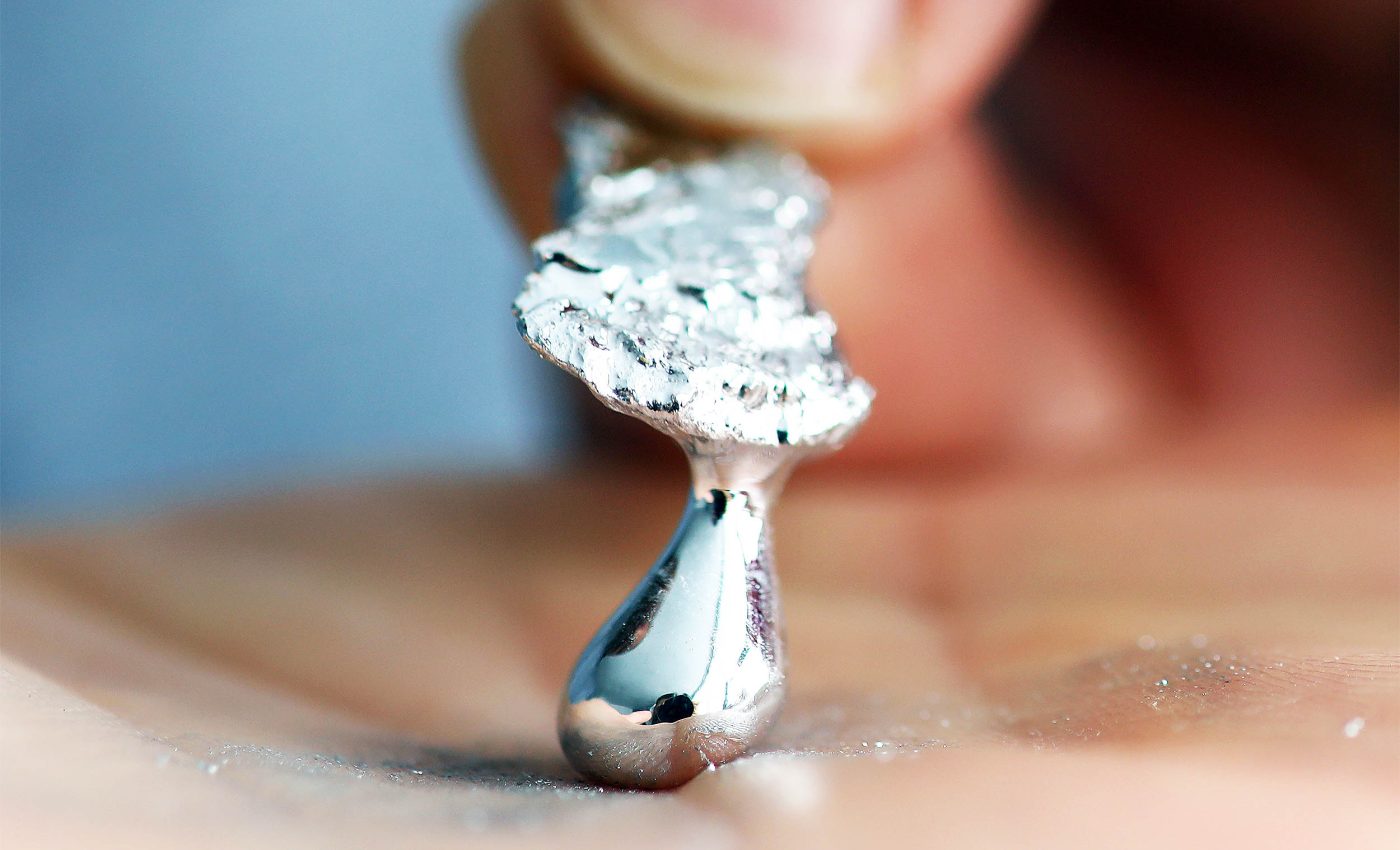
A spoon made of gallium will liquefy in a cup of tea, yet the same element anchors semiconductors, solar panels, and laser diodes that withstand scorching factory conditions.
For more than 30 years, physicists have wrestled with gallium’s structure in the liquid state. Competing studies have offered clashing explanations, especially around the role of covalent bonding – a type of electron-sharing connection that metals rarely show.
Fresh simulations have now stitched the puzzle pieces together and point to what scientists believe to be the decisive proof they’ve been searching for.
Why gallium melts early
Gallium melts at 85 °F, far below the temperature of a warm summer day. That quirky transition happens because solid gallium forms tiny two-atom pairs called dimers, and those pairs pack less densely than the liquid. The moment the dimers break apart, density rises and the metal flows.
The story becomes stranger at higher heat. At temperatures well above the phase change, the dimers re-form, but not in ways scientists expected.
“Thirty years of literature on the structure of liquid gallium has rested on a fundamental assumption that is evidently not true,” says Professor Nicola Gaston of Waipapa Taumata Rau, University of Auckland, and the MacDiarmid Institute for Advanced Materials and Nanotechnology.
Her team combined ab initio molecular-dynamics data with decades of experimental records to see how individual atoms mingle at several checkpoints on the thermometer.
Liquid gallium myths overturned
Conventional wisdom held that covalent bonds fade out once gallium liquefies and play only a minor role afterward.
The fresh computational work shows the opposite: covalency matters more as the temperature climbs. In other words, the tighter electron sharing that disappears at 85 °F re-emerges and strengthens when the metal is pushed hundreds of degrees hotter.
The insight explains a long-standing electrical oddity. Gallium’s resistivity dips as it first passes the melting line – counter to most metals – then creeps upward in a nonlinear fashion.
If covalent bonds regroup at elevated heat, they create a conduction bottleneck that raises resistivity, squaring experimental curves with atomic-level theory.
Textbook melting points depend on the tug-of-war between internal order and random motion. When the dimers dissolve, entropy shoots up, giving atoms the freedom to shift.
That entropy spike accounts for gallium’s unusually low melt point without invoking mysterious bonding tricks at 85 °F.
Once the liquid sits comfortably above the transition, the atomic sea settles just enough for bonds to snap back together – only this time within a looser, more disordered framework.
Zinc crystals from liquid gallium
Gallium’s renewed covalency at high temperatures also clarifies why the liquid can pull other metals into solution with minimal energy input.
The shared-electron environment encourages elements such as zinc, copper, and tin to mix, forming room-temperature liquid alloys that engineers mold into stretchable circuits, heat-free solders, and microfluidic pumps.
A few years ago, researchers crystallized zinc inside a bath of molten gallium and watched delicate six-sided plates snowball outward. That project hinted at liquid gallium’s knack for directing complex shapes without external templates.
The new structural roadmap shows that covalent rebonding supplies the subtle forces that align dissolved atoms as they solidify, making it easier to predict – and exploit – growth patterns.
Gallium-based liquids already act as catalysts that shuffle reactants into greener chemical pathways. The updated model will help chemists tune alloy recipes for faster charge transfer or sharper selectivity.
In battery prototypes, thin films of gallium alloys spread across electrodes, filling microscopic cracks that normally kill capacity. Knowing when the liquid switches bonding modes could lengthen battery lifetimes and lower manufacturing temperatures.
Quirks in fabrication
Gallium’s solid compounds, including gallium arsenide and gallium nitride, drive radio-frequency amplifiers, high-brightness LEDs, and fast chargers.
Fabricating those compounds sometimes involves droplets of pure gallium that remove heat or transport precursor atoms.
Process engineers need precise viscosity, density, and conductivity data at every stage, and the re-emergence of covalent links at high heat feeds directly into those parameters.
Understanding gallium’s behavior also safeguards supply chains. The metal is mined as a by-product of aluminum production, mainly from bauxite ore.
Global output hovers around 460 tons per year, and demand surges whenever display or photovoltaic markets expand. Tailoring alloys that use less gallium, yet keep its liquid perks, will ease strain on future inventories.
Why does any of this matter?
Beyond Earth, gallium could help planetary scientists sniff out ancient microbes. Because the element forms stable bonds with oxygen and sulfur, it may lock biosignatures into Martian rock layers.
Some geochemists argue that gallium traces could outlast fragile carbon compounds, providing a longer-lived clue to life. The revamped structural picture supplies the chemical logic behind that durability.
As liquid metals step into flexible electronics, soft robotics, and low-carbon chemistry, knowing exactly how gallium’s atoms dance under heat will pay dividends.
Gallium still bends spoons for party tricks, but its greater trick lies in how a simple temperature dial rewrites atomic rules.
By showing where covalent bonds vanish and return, the research clears fog from a field that fuels countless technologies, from phone screens to satellite links.
The full study was published in the journal Materials Horizons.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













