Technological leap turns carbon dioxide (CO2) into methanol fuel
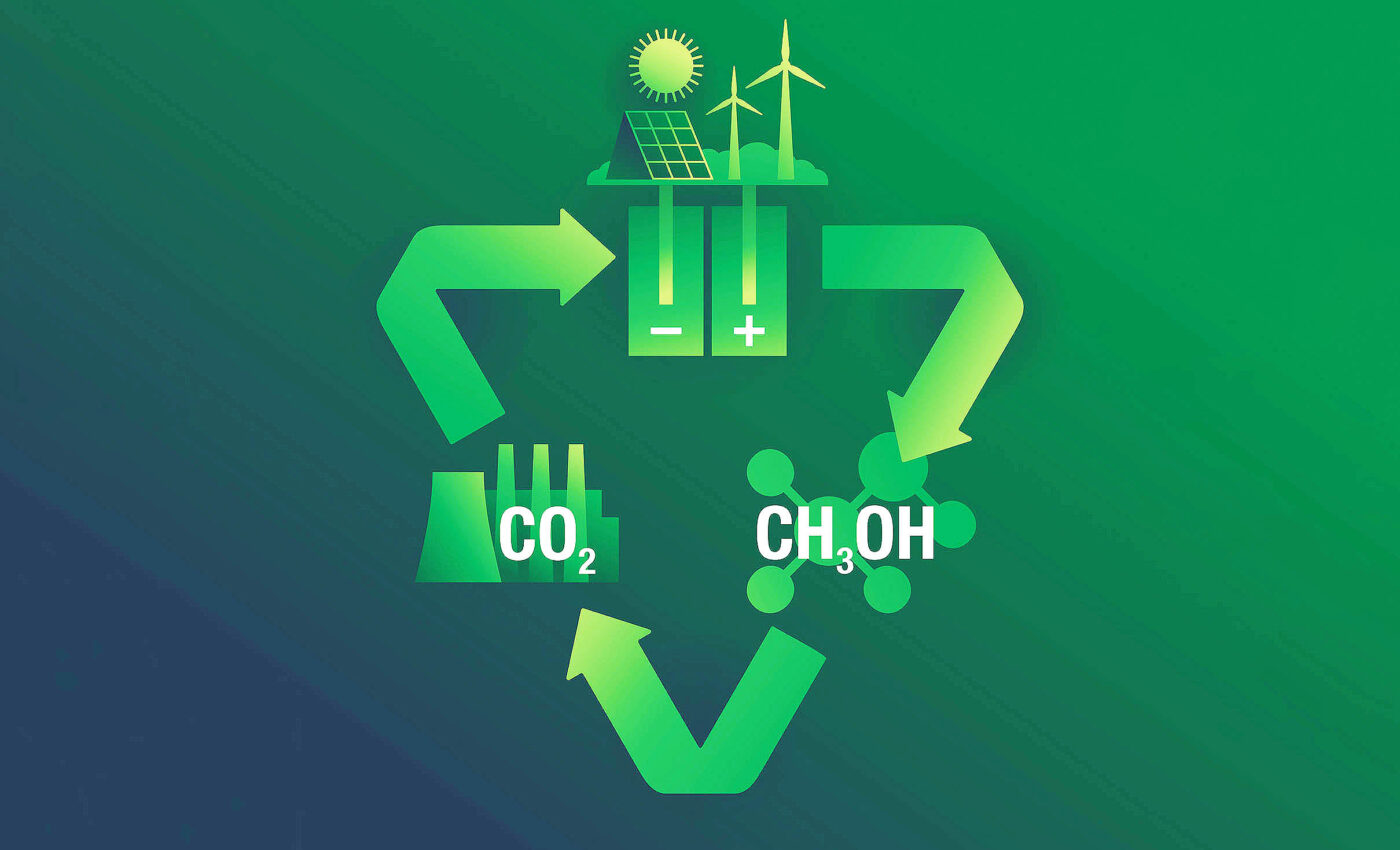
In an era where climate change poses significant challenges, an exciting development in renewable energy has emerged. Researchers have discovered a way to convert carbon dioxide (CO2) into methanol, offering a pathway to reduce greenhouse gas emissions and move closer to achieving carbon neutrality.
A team of dedicated researchers at the University of Michigan has unveiled a novel catalyst material, cobalt phthalocyanine, which facilitates this transformative process.
This innovative catalyst efficiently converts CO2 — a major contributor to global warming — into methanol, providing a sustainable alternative for energy production.
Methanol production via CO2
Published in the journal ACS Catalysis, this research showcases the multi-step chemical reaction facilitated by cobalt phthalocyanine.
Initially, CO2 is converted into carbon monoxide (CO), which is then transformed into methanol. These reactions demonstrate not only the catalyst’s effectiveness but also its potential for large-scale applications.
The method is distinguished by its sustainable approach to reducing emissions while simultaneously generating clean energy.
The capability to chemically convert CO2 into methanol is especially notable, considering methanol’s potential as a cleaner fuel alternative that could power vehicles with a significantly lower environmental impact.
Challenges in producing methanol from CO2
While the industrial conversion of CO2 to methanol is not new, the scale and efficiency of these processes through electrochemical means have posed substantial challenges. Kevin Rivera-Cruz, a recent doctoral graduate in chemistry is a co-primary author of the study.
“Our approach is unique because we are able to bring and bridge all this knowledge that each field has on the same problem. We have scientists and engineers all within one team, brainstorming and gathering insights to design and understand the system in the best way possible,” Rivera-Cruz explained.
Overcoming scientific hurdles
The researchers encountered a significant hurdle: cobalt phthalocyanine’s tendency to bind more strongly with CO2 than with CO.
This strong binding to CO2 leads to its displacement by another CO2 molecule before CO can be converted to methanol.
To address this, the team utilized advanced computational modeling and experiments to delve deeper into how the catalyst’s electrons interact with both CO2 and CO molecules. Their findings indicated that cobalt phthalocyanine binds CO2 over three times more tightly than it does with carbon monoxide.
Armed with this knowledge, the researchers propose redesigning the catalyst to enhance its interaction with CO and reduce its affinity for CO2.
Such modifications are crucial for the efficient conversion of CO2 to methanol on a large scale, paving the way for the practical use of this technology in renewable energy applications.
Methanol as a pathway to sustainability
The journey toward a carbon-neutral future is fraught with scientific and technological challenges. Achieving carbon neutrality requires reducing our reliance on fossil fuels and finding innovative ways to mitigate greenhouse gas emissions. However, the path is filled with complex obstacles, from developing efficient technologies to scaling them for widespread use.
Despite these challenges, researchers continue to make significant strides through innovations like cobalt phthalocyanine. This novel catalyst material efficiently converts CO2, a leading contributor to climate change, into methanol.
Methanol is a versatile fuel alternative with a significantly lower environmental impact. By addressing the dual challenge of reducing CO2 emissions and providing cleaner energy, this innovation offers a promising solution.
By redesigning the way we handle waste CO2 and converting it into useful methanol fuel, we edge closer to a sustainable and energy-secure world. This new approach to recycling CO2 mitigates emissions and creates a valuable product that can power vehicles and industries in a more environmentally friendly way.
The full study was published in the journal ACS Catalysis.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













