Ancient phage viruses may hold the key to defeating superbugs
The fight against bacterial infections is becoming harder. Across hospitals, some illnesses no longer respond to standard drugs, and doctors are forced to rely on last-line treatments.
As bacteria evolve past antibiotics, attention is shifting toward viruses that specialize in hunting them – bacteriophages, or phages for short.
These are viruses that infect bacteria, not people or animals. They exist in soil, oceans, and inside our bodies, and scientists are testing how to turn them into highly precise antibacterial tools.
What makes phages unique
In a recent study led by a team at the University of Otago’s Department of Microbiology and Immunology, together with colleagues at the Okinawa Institute of Science and Technology (OIST), researchers focused on one of these viruses in intense detail.
Lead author Dr. James Hodgkinson-Bean noted that bacteriophages are “extremely exciting” in today’s scientific world.
Researchers see them as promising antibiotic alternatives as the risk of antimicrobial resistance continues to rise.
“Bacteriophage viruses are non-harmful to all multicellular life and are able to selectively target and kill a target bacterium,” he said.
“Due to this, they are increasingly being researched and applied in ‘phage therapy’ to treat highly drug-resistant bacteria.”
Dr. Hodgkinson-Bean describes bacteriophages as “exquisitely intricate viruses” that infect bacteria through large mechanical structures described as “tails.”
These tails lock onto a bacterial cell and trigger a chain of events that allow the virus inject its genetic material.
That precision is why researchers view these viruses as custom tools, each phage matched to a single type of bacterium.
Bacteriophages vs. E. coli
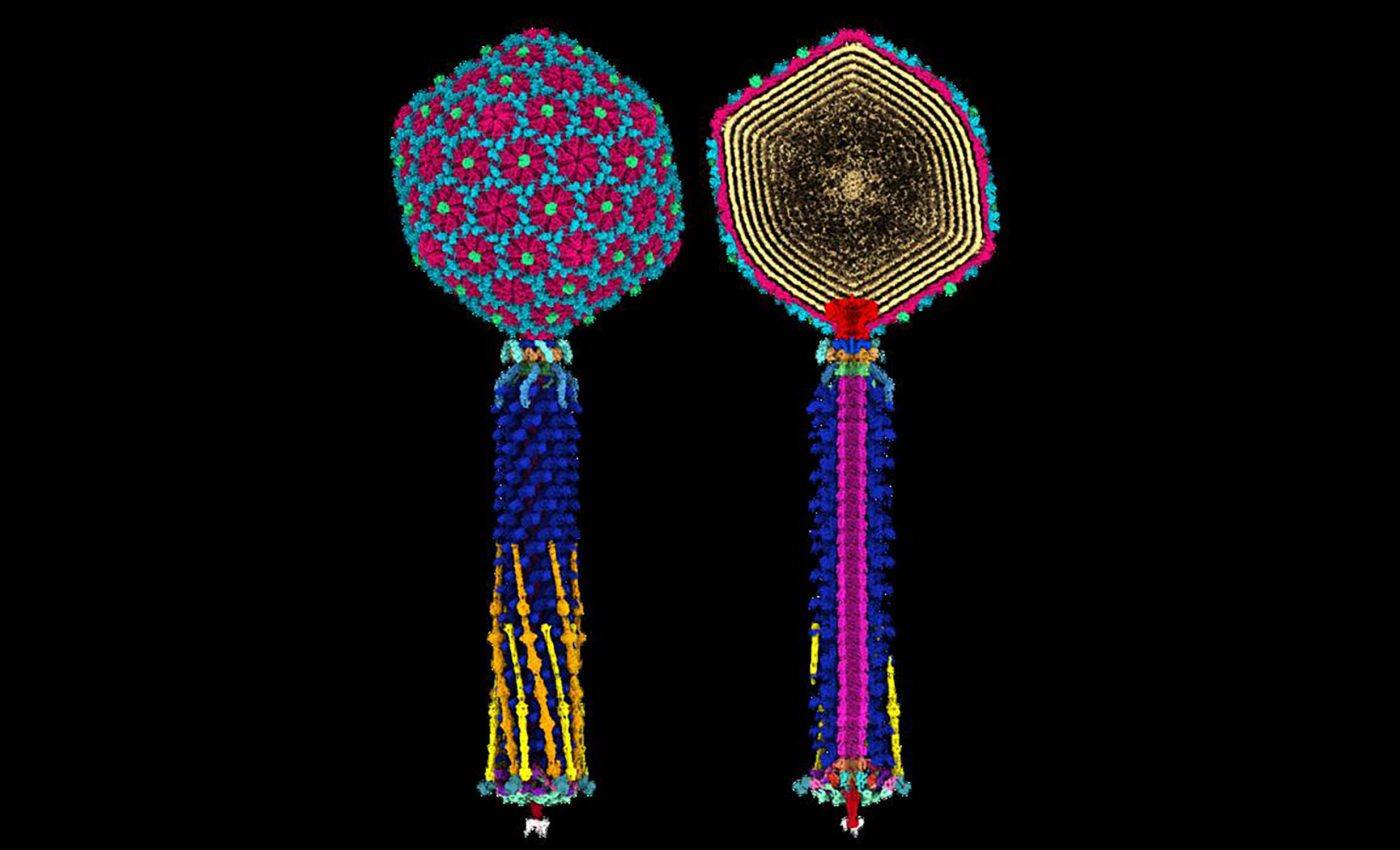
For this study, the team explored the structure of Bas63, a virus which targets E. coli, in molecular detail to understand how its tail works during infection.
E. coli is one of the best-known bacteria in biology, with harmless strains in our gut and dangerous strains that cause severe illness. A virus that attacks it therefore offers a sharp test case for therapy.
Bas63 belongs to a family of phages with elaborate tail machinery. When it finds an E. coli cell, proteins on the tail recognize features on the bacterial surface.
Once attached, the tail rearranges like a tiny molecular device, pushing the virus’s DNA into the cell.
Mapping each part of that system helps explain why some phages infect only very specific strains while others have a broader reach.
Phage virus blueprint in 3D
Senior author Mihnea Bostina, an associate professor in Otago’s Department of Microbiology and Immunology, links this structural work to real-world uses.
Professor Bostina pointed out that with antibiotic resistance rising and plant pathogens threatening global food security, bacteriophages offer a promising alternative.
“Our detailed blueprint of a bacteriophage advances rational design for medical, agricultural, and industrial applications, from treating infections to combating biofilms in food processing and water systems,” he said.
“Beyond science, the 3D data – which shows the virus’s rare whisker-collar connections, hexamer decoration proteins, and diverse tail fibers – may inspire artists, animators, and educators.”
Researchers generate this kind of blueprint using high-resolution imaging, often by freezing samples and capturing thousands of particle pictures at different angles.
Computer tools combine those pictures into a 3D model. This lets scientists see how each protein fits into the overall structure and how the tail elements move when the virus switches from searching to attacking.
Evolution through viral design
“While DNA generally serves as the best evolutionary marker in humans, the 3-dimensional structure of a virus is more informative of its distant evolutionary relationships with other viruses,” said Dr. Hodgkinson-Bean.
The experts found features previously seen only in very distantly related viruses, revealing previously unknown evolutionary links between them.
Structural patterns can persist even when genetic sequences change. Viral genomes often swap segments, lose genes, or pick up new ones from hosts and other viruses, which can blur long-term relationships.
Architecture, like the layout of the capsid and tail parts, can stay surprisingly stable across huge spans of time. Comparing these 3D shapes lets researchers track those ancient lines.
Living fossils in the virus world
Dr. Hodgkinson-Bean connects these structural clues to a much deeper timeline.
“We know through structural studies that bacteriophages are related to herpes viruses,” he said. “This relationship is thought to extend back billions of years to before the emergence of multicellular life.”
“For this reason, when we look at bacteriophage structure, we are looking at living fossils – primordial ancient beings. There is something truly beautiful about that.”
Seeing those links gives researchers a way to reconstruct how today’s viral groups emerged from older forms. It shows that modern medical tools can trace their roots to life’s earliest stages, long before animals or plants existed.
Phage viruses in crops and industry
The potential uses of phages stretch beyond human medicine. The researchers pointed out that plant disease is a major threat to crops.
The experts noted that phages can be tuned to attack specific bacterial pests without harming beneficial microbes. This makes them interesting for agriculture, where selective treatments are important for soil health and long term productivity.
“This kind of research is important for understanding how we can select the optimal bacteriophages for therapies, and to understand the differences in infectious behavior we see in the lab,” said Dr. Hodgkinson-Bean.
By tying behavior to structure, teams can sort through candidate phages. They can then match them to medical, agricultural, or industrial tasks where their strengths matter.
Isolating viruses with phage therapy
In clinics, phage therapy often starts with isolating viruses that attack the strain infecting a patient, then combining several into a tailored mix.
Because phages replicate only where their target bacteria live, they can increase in number right at the infection site.
This behavior is very different from how standard drugs work. That adaptability may help as part of combination treatments alongside antibiotics.
As more detailed virus blueprints like the Bas63 model appear, designers can start to think in a more systematic way about which phages to use. They can also plan how to monitor their effects and how to respond when bacteria change again.
The work brings together structural biology, evolutionary thinking, and practical problem-solving. It centers on tiny viruses that have been on Earth for billions of years and might help tackle one of today’s toughest health challenges.
The full study was published in the journal Science Advances.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













