Why is the Nitrogen Cycle So Important?
Nitrogen might not get spoken about as much as other atmospheric chemicals like oxygen or carbon dioxide, but that doesn’t mean it is any less important. In fact, all life on Earth requires nitrogen to survive. The nitrogen cycle is one of the most useful biogeochemical cycles to understand.
Why does all life require nitrogen?
Plants and photosynthetic bacteria are the primary producers of this planet. That means they are the living things that convert sunlight into energy. All other life benefits from this process when energy moves up the food web.
Primary producers need a lot of nitrogen to be able to convert sunlight into energy. Chlorophyll is the part of a cell in these primary producers that conducts the process of photosynthesis. Chlorophyll takes a lot of nitrogen to create. So if nitrogen is limited, which it often is, then chlorophyll is also limited. Limited chlorophyll means limited photosynthesis, which means less overall energy in the system for life to benefit from.

Why is Nitrogen Limited?
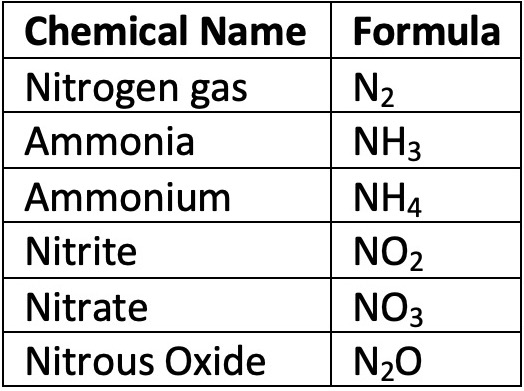
Surprisingly, the air we breathe is only about 20% oxygen. The rest of air, almost 80%, is nitrogen gas, or N2. If there is so much nitrogen in the atmosphere, why is it often the limiting factor in plant growth?
Plants can’t utilize gaseous nitrogen. Instead, plants need nitrogen in the form of nitrate (NO3) or ammonium (NH4).
Imagine you were in a European country that only used Euros. You wouldn’t be able to use American dollars to pay for goods. To utilize your dollars, you would need to visit a bank to convert the dollars into Euros. From there, you would be able to buy baguettes, tomatoes, and cheese.
If the dollars are N2 gas and the Euros are nitrate and ammonium, what process in nature functions like the bank in this metaphor? What converts atmospheric nitrogen gas into usable ammonium and nitrate?
Understanding the nitrogen cycle will help you answer this question.
The Five Steps of Nitrogen Cycling
The nitrogen cycle is complex and multi-faceted. This section will briefly cover the five main processes in the cycle without focusing on the chemistry too much.
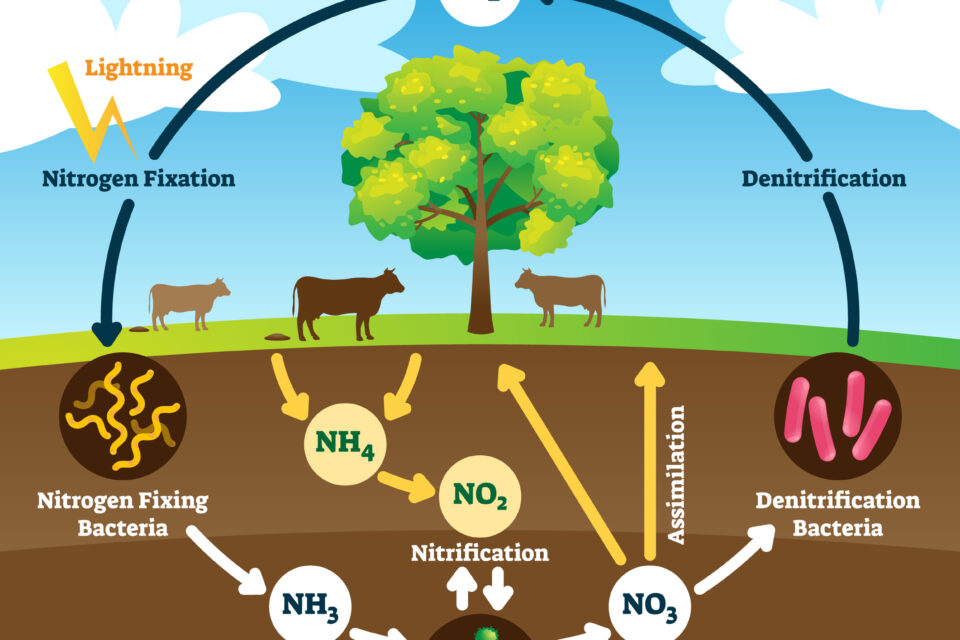
1: Nitrogen Fixation
Nitrogen fixation is the process by which atmospheric nitrogen converts to ammonium. Nitrogen fixation is the equivalent of the bank in the metaphor above.
Tiny bacteria and archaea do this important job. There are two main microbial groups that fix nitrogen. One group does the job without help from other organisms. These bacteria are called free-living bacteria. All ocean-dwelling, nitrogen-fixing bacteria are free-living. The second group associates with plant roots in a symbiotic relationship to fix nitrogen (more on them later).
All of these special bacteria contain an enzyme called nitrogenase. Nitrogenase is the only enzyme known to change N2 to ammonia (NH3). Under normal conditions, the bond between the two nitrogen atoms in N2 is strong and difficult to break. Nitrogenase decreases the amount of energy required to break the triple bond of the N2, which allows these nitrogen-fixing bacteria to turn N2 into ammonia (NH3) or ammonium (NH4).
Can Plants Use Ammonium?
Plants can use fixed nitrogen like ammonium as their nitrogen source, but it is not the best source. Plants rapidly suck up ammonium when it is applied to the soil. However, ammonium is toxic to plants. Plants can convert ammonium to non-toxic forms of nitrogen, but only at a certain speed. If a plant sucks up ammonium faster than it can convert ammonium to non-toxic nitrogen, the plant will start to die.
For these reasons, we should be thankful for the next step in the nitrogen cycle, which is nitrification.
2: Nitrification
Once bacteria have turned atmospheric nitrogen into ammonium, different nitrifying bacteria turn the ammonium into nitrite (NO2) through oxidation. After the nitrogen turns into nitrite, yet different species of bacteria turn it into nitrate (NO3). Nitrate is a more stable molecule than nitrite. Nitrate is the best source of nitrogen for primary producers. On land, these bacteria live in the soil. In water, these bacteria float freely.
Nitrification happens in an aerobic environment, meaning that oxygen is present.

3: Assimilation
The third step in the nitrogen cycle is where primary producers ingest nitrogen into their cells. Since nitrate, unlike nitrogen gas, is a form of nitrogen that plants can ingest, plants will uptake as much nitrate as possible. Plants assimilate nitrogen into their cells via roots. The roots then transport the nitrate to the cells where it is needed.
Living organisms require nitrogen for many processes. Most fundamentally, life requires nitrogen as an important part of amino acids and nucleic acids. Amino acids are the building blocks of all proteins.
Not all nitrogen gets assimilated into living beings. In the ocean, some nitrates fall to the ocean bottom as sediment. This sediment can, over geologic time, undergo mineralization to create sedimentary rock.
Plants Don’t Always Assimilate the Maximum Amount of Nitrogen
While nitrogen is often the limiting nutrient for plants, phosphorus can also be a limiting nutrient. If phosphorus is the limiting nutrient, the plant won’t be able to assimilate any more nitrate.
Check out a bag of fertilizer next time you see it in the store. The three numbers on the front mean the ratio of nitrogen to phosphorus to potassium, or N-P-K. You will notice that most of these fertilizers have more nitrogen and phosphorus than potassium. That’s because potassium is less likely to be the limiting nutrient.
Limiting factors other than nutrients can be sunlight and water.

4: Ammonification
Nothing lives forever! When a living being dies, its cells decay with the help of decomposers, like bacteria and fungi. The nitrogen in these dead cells doesn’t simply disappear. The decaying bacteria and fungi turn the nitrogen in the decaying cells back into ammonium. This ammonium will likely go back to step 2 in the nitrogen cycle.
The nitrogen that comes from decomposing organic matter is called organic nitrogen. Nitrogen that does not is called inorganic nitrogen.
Human and animal poop also decays through ammonification.
5: Denitrification
Denitrification is an alternative route through the nitrogen cycle. Instead of nitrate being assimilated into primary producers (step 3), it might jump straight to denitrification. Denitrification happens in anaerobic environments. Anaerobic environments are environments that are very low in oxygen. Instead of using the oxygen present in air for respiration, like us humans do, microbes in anaerobic environments steal oxygen off of oxygen-rich molecules for respiration. Nitrate (NO3) has three oxygen atoms, making it a great option for anaerobic respirators.
Various species of denitrifying bacteria that operate in anaerobic environments break down nitrate (NO3) to nitrite (NO2) to nitric oxide (NO) to nitrous oxide (N2O) and finally back to nitrogen gas (N2).

Climate Implications of Denitrification
Nitrous oxide is a greenhouse gas 300 times more powerful than carbon dioxide. Because of this, the nitrogen cycle has big impacts on anthropogenic climate change. Approximately 7% of U.S. greenhouse gas emissions are nitrous oxide. Human activities around the world, such as agriculture, animal agriculture, sewage treatment, and fossil fuel combustion produce 40% of the world’s nitrous oxide. Agricultural practices account for about 75% of human-caused nitrous oxide.
Other Natural Sources of Nitrogen Fixation
There are three sources of natural nitrogen fixation. Bacteria, as shown above, are the major natural nitrogen fixers. These microorganisms fix about 90-95% of the natural nitrogen. Nitrogen fixation done by living organisms is called biological nitrogen fixation. Bacteria do this via the nitrification processes described above using the enzyme nitrogenase. There are a couple of other noteworthy types of natural nitrogen fixation.

Legumes
Some important nitrogen-fixing bacteria have evolved a symbiotic relationship with plants in the legume family. Plants in this family include peas, soybeans, beans, and peanuts. These plants have root nodules that look like funny balls on the roots. These balls provide housing for nitrogen-fixing bacteria. In return for housing, these bacteria fix nitrogen from the atmosphere and give it straight to their host plant.
Growing legumes is a great way to naturally build up nitrogen in the soil. When the legume plants die, the decomposing bacteria and fungi return the nitrogen from those dead legumes into the soil. Once in the soil, any kind of plant can use the recycled nitrogen from the decayed legumes.

Lightning
Lightning accounts for nearly all of the inorganic, natural nitrogen fixation. This is because lightning has enormous amounts of energy and it always travels through air, which is 80% nitrogen. This means lightning always comes into contact with a lot of nitrogen. Lightning heats the air surrounding it to about 50000F. That’s five times hotter than the surface of the sun!
The super-hot lightning breaks the strong bonds in atmospheric nitrogen gas. Once those bonds are broken, the nitrogen atoms quickly cool off. As they cool, the solo nitrogen atoms recombine with the oxygen present in the air to make nitrite. The nitrite then clings on to moisture in the air and turns into nitrate. When that moisture falls to the ground as rain, the nitrate hitches a ride down to Earth. Rain during a lightning storm literally rains fertilizer!

Volcanoes
Volcanoes are the least significant natural source of nitrogen fixation. They currently produce a relatively insignificant amount of nitrate. Scientists don’t fully understand the process of volcanic nitrification. It seems that the magma heats the air similarly to lightning. As that air rises in the smoke plume, it cools off and the nitrogen combines with oxygen.
Volcanoes and lightning don’t produce anywhere near the majority of nitrate on Earth. This hasn’t always been the case. Way back when nitrogen-fixing bacteria did not exist. Volcanoes and lightning were the only sources of biologically usable nitrogen for 2 billion years. The nitrogen that lightning and volcanoes fixed made the evolution of nitrogen-fixing bacteria possible. Without volcanoes and lightning, life wouldn’t exist on planet Earth.
Human Impacts on the Nitrogen Cycle
Like just about everything else on planet Earth, humans have had a significant impact on the nitrogen cycle. Amazingly, humans create about the same amount of nitrate and ammonium as all of nature. Most of this nitrate comes from an industrial chemical process called the Haber process.
Humans use nitrate and ammonium extensively in agriculture. We also produce nitrate with our sewage, especially in animal agriculture. The combustion of fossil fuels also impacts the nitrogen cycle.

Haber Process
The Haber process is arguably one of the most important human discoveries of all time. It is definitely the most important discovery in terms of the nitrogen cycle. This process synthesizes ammonia from air and methane. Fritz Haber and Carl Bosch discovered the technique in 1909.
Burning methane releases hydrogen gas. In the Haber process, hydrogen gas is fed into a chamber with air. When put under great amounts of pressure, the strong nitrogen gas bonds in air will break. These nitrogen molecules then recombine with hydrogen to make ammonia. Ammonia can then be turned into nitrate relatively easily. Haber and Bosch changed the course of world history by discovering how to synthesize biologically usable nitrogen from air and methane.
How Did the Haber Process Change World History?
Nitrogen is a major factor that limits plant growth. Up until the Haber process enabled synthetic fertilizers, farmers relied on animal waste as their main fertilizer. However, there was never enough animal waste to grow crops to their biggest yields.
Adding biologically available nitrogen to agricultural fields increases crop yields. Bigger crop yields increase the number of people an acre of farmland can feed. More fertilizers created more food.
Put simply, the Haber process allows humans to grow more food. Without nitrate from the Haber process farmers would have a much more difficult time feeding the world. This process is one of the leading reasons for why the human population exploded from two billion to nearly eight billion in the last 100 years. The Haber process changed world history by enabling this population explosion.

The Haber Process Requires Massive Amounts of Energy
Manufacturing ammonia via the Haber process accounts for 1.2% of all greenhouse gas emissions. This amount of emissions is equivalent to the emissions from almost 100 million cars. These emissions come directly from burning methane and indirectly from the power plants that supply electricity to ammonia manufacturing facilities.
Creating high-pressure chambers for nitrogen gas to bond with hydrogen gas requires massive amounts of energy. The Haber process also consumes about 1% of global electricity.
Chemists around the world are working on less carbon-intensive ways of manufacturing ammonia. One method tries to imitate lightning. As you can imagine, making lightning is also incredibly energy-intensive. As this research progresses, chemists hope that they can figure out a more environmentally friendly way to make ammonia.

Agriculture
Agriculture is a main way that humans have impacted the nitrogen cycle. The majority of ammonia created through the Haber process is used as agricultural fertilizer. Farmers around the world routinely apply nitrate to their fields to increase crop yields. While this supplemental nitrogen boosts yields, it is not without consequences.
Plants don’t suck up all of the nitrate applied to fields. This may be because the farmer applied too much fertilizer. It could also be because rain washed the nitrates off the field. Lastly, it could be because irrigation pushed the nitrate deep into the soil beyond the root zone of the plants.

When There is Too Much Nitrogen
Whatever the reason, nitrate that isn’t used by plants becomes agricultural runoff. Since nitrates are very soluble in water, runoff concentrates nitrates in streams. Often, many streams with nitrate runoff feed into a single lake. While nitrogen is beneficial for life in many circumstances, there is such a thing as too much nitrogen. Too much nitrogen can fundamentally change the ecology of a place.

Eutrophication
Putting too much nitrogen in an ecosystem can throw the entire ecosystem off balance. When a body of water has too much nitrogen, the photosynthetic algae grow like crazy. The growth of this algae is typically limited by the amount of nitrogen present in the water. When agricultural runoff provides the algae with ample nitrogen, the algae can grow many times faster than it naturally would creating algal blooms. If you’ve ever seen a scummy, green lake, it is probably due to nitrogen contamination.
This algae eventually dies. Bacteria consume the dead algae. In order to consume the dead algae, bacteria also consume dissolved oxygen in the water. This oxygen consumption leads to lower oxygen levels in the water. Many species of fish and freshwater invertebrates require a specific amount of dissolved oxygen to survive. When the oxygen drops below that level, there can be mass die-offs of fish. Just as humans wouldn’t live very well if the amount of oxygen in our atmosphere was depleted, fish don’t do very well if the amount of oxygen in their lakes and streams disappears. To add insult to injury, these same bacteria emit large amounts of methane, a potent greenhouse gas.

The word ‘eutrophic’ means ‘rich in nutrients’. Eutrophication is the process described above.
Rivers can also become eutrophic. However, this is less common because rivers are constantly moving whereas lakes are more stagnant. That stagnation creates the perfect environment for algae to grow when given ample nitrogen.
Eutrophication also decreases the recreational value of waters by making them unsightly and smelly.

Estuaries
Much of the nitrogen that pollutes lakes and streams will eventually reach the ocean by rivers. This large deposit of nitrogen in coastal waters and estuaries also causes eutrophication. 65% of estuaries and coastal waters in the continental United States are contaminated by excess nutrients. Eutrophication of marine ecosystems negatively impacts commercial fisheries in the same way as it does in lakes. Imagine all of the nitrogen that gets carried down the Mississippi River and deposited in the delta near New Orleans. The Mississippi River carries about double the amount of nutrients it did 100 years ago. The nutrient runoff in Iowa impacts fisherpeople all the way off the coast of Louisiana.
Nitrogen pollution from human activities from terrestrial ecosystems can even reach the open ocean.

Animal Agriculture
Animal agriculture is the biggest contributor to the eutrophication of lakes and rivers. The state of Iowa, for example, introduces 400 million pounds of nitrogen into the nitrogen cycle every year through animal waste. A dairy operation with 200 cows can produce the same amount of nitrogen waste as a city of 10,000 people. A single cow produces almost half a pound of pure nitrogen in its feces every day.
Animal waste concentrates nitrogen in the forms of urea and ammonia. Some animal manure is used to fertilize crop fields. The application of animal manure on fields can lead to nitrogen runoff as described above. This runoff is especially bad when there is high-intensity, concentrated agriculture. This concentration of agriculture leads to a concentration of nitrogen in waterways.
While manure is a valuable fertilizer, concentrated animal feed operations (CAFOs) produce too much manure in one area for farmers to handle. CAFOs produce thousands of pounds of manure per day. Typically, there aren’t enough farmers nearby to use the manure produced by these operations.
Instead of using manure as fertilizer, CAFOs will repetitively spread manure on ‘spray fields’. This process quickly overloads the soil with nitrogen and makes it easy for the nitrogen to runoff into rivers.
CAFOs also store manure in ‘treatment lagoons’. This option is prone to failure, especially during flooding. A single hog operation in North Carolina has spilled manure into the surrounding watershed over 40 times.

Wastewater
The waste from animals produces many times more nitrogen pollution than human waste. That being said, human waste can significantly deteriorate rivers and lakes if sewage isn’t properly treated. Megalopolis cities, such as Mexico City and Tokyo, have upwards of 30 million people. All those people produce sewage that is high in nitrogen. Ideally, sewage treatment plants use various techniques to remove nitrogen from the sewage as they treat it. This way, when the treated sewage is put back into the environment it doesn’t contribute to eutrophication.

Fossil Fuels
The combustion of fossil fuels also impacts the nitrogen cycle. Burning fossil fuels creates nitrous oxides. These nitrous oxides are gases that exist in the earth’s atmosphere and negatively impact air quality by creating smog. Bad nitrous oxide pollution creates serious respiratory problems for humans. These nitrous oxides, as mentioned above, are potent greenhouse gases. The nitrous oxides fall back down to Earth in rain, which can cause acid rain. This rain acidifies the soil and reduces its fertility.
Catalytic converters substantially reduce the nitrous oxides emitted by vehicles. These converters split the NO2 into nitrogen and oxygen gases, which are already present in our atmosphere.
Final Thoughts
Nitrogen is an essential nutrient for life on Earth. It provides the nutrient necessary for photosynthesis. All life depends on photosynthesis to thrive. Because nitrogen is so crucial for our biosphere, humans have figured out clever ways to create an abundance of it.
As discussed, nitrogen can also be toxic when there is too much of it in the ecosystem. Worldwide nitrate reduction would result in positive impacts for our air, climate, and water. Smarter application of fertilizers to crops, better management of animal waste, and stronger sewage treatment and automobile exhaust standards could all significantly decrease the negative impacts humans have on the global nitrogen cycle.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.